当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel benzimidazole-linked (thio)barbiturates as non-hydroxamate HDAC6 inhibitors targeting leukemia: Design, synthesis, and structure–activity relationship
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2023-03-21 , DOI: 10.1002/ardp.202200433 Reda El-Sayed Mansour 1 , Hanan Gaber Abdulwahab 1 , Hend M El-Sehrawi 1
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2023-03-21 , DOI: 10.1002/ardp.202200433 Reda El-Sayed Mansour 1 , Hanan Gaber Abdulwahab 1 , Hend M El-Sehrawi 1
Affiliation
|
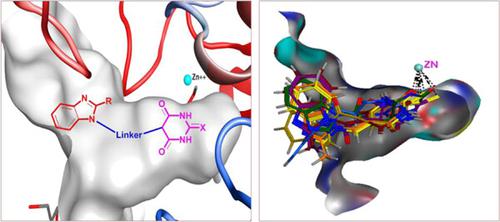
Based on the well-established pharmacophoric features required for histone deacetylase (HDAC) inhibition, novel easy-to-prepare benzimidazole-linked (thio)barbiturate derivatives were designed and synthesized as HDAC6 inhibitors. The proposed structures of the title compounds were confirmed based on their spectral data and elemental analyses. The newly synthesized compounds were screened in vitro against HDAC6. All tested compounds showed potent HDAC6 inhibition at the nanomolar level. Several compounds displayed a remarkable HDAC6 inhibitory activity (IC50 = 48.85–75.62 nM), superior to that of the reference drug suberoylanilide hydroxamic acid (SAHA; IC50 = 91.73 nM). The most potent derivatives were further assessed for their in vitro anticancer activity against two human leukemia cell lines. Thiobarbiturate 3e was two times more potent than SAHA against the tested cells. The detailed structure–activity relationship was also described. Furthermore, molecular docking simulation revealed the ability of the title compounds to chelate the catalytic Zn+2 ion located within the binding pocket of HDAC6. In silico evaluation of physicochemical properties indicated that the target compounds are promising candidates in terms of pharmacokinetic aspects.
中文翻译:

新型苯并咪唑连接的(硫代)巴比妥酸盐作为针对白血病的非异羟肟酸盐 HDAC6 抑制剂:设计、合成和构效关系
基于组蛋白脱乙酰酶 (HDAC) 抑制所需的成熟药效团特征,设计并合成了新型易于制备的苯并咪唑连接的(硫代)巴比妥酸盐衍生物作为 HDAC6 抑制剂。标题化合物的拟议结构基于其光谱数据和元素分析得到确认。新合成的化合物在体外针对 HDAC6 进行了筛选。所有测试的化合物都显示出纳摩尔水平的强效 HDAC6 抑制作用。几种化合物表现出显着的 HDAC6 抑制活性(IC 50 = 48.85–75.62 nM),优于参考药物辛二酰苯胺异羟肟酸(SAHA;IC 50 = 91.73 纳米)。进一步评估了最有效的衍生物对两种人类白血病细胞系的体外抗癌活性。硫代巴比妥酸盐3e对测试细胞的效力是 SAHA 的两倍。还描述了详细的构效关系。此外,分子对接模拟揭示了标题化合物螯合位于 HDAC6 结合口袋内的催化 Zn +2离子的能力。物理化学性质的计算机评价表明,目标化合物在药代动力学方面是有前途的候选者。
更新日期:2023-03-21
中文翻译:

新型苯并咪唑连接的(硫代)巴比妥酸盐作为针对白血病的非异羟肟酸盐 HDAC6 抑制剂:设计、合成和构效关系
基于组蛋白脱乙酰酶 (HDAC) 抑制所需的成熟药效团特征,设计并合成了新型易于制备的苯并咪唑连接的(硫代)巴比妥酸盐衍生物作为 HDAC6 抑制剂。标题化合物的拟议结构基于其光谱数据和元素分析得到确认。新合成的化合物在体外针对 HDAC6 进行了筛选。所有测试的化合物都显示出纳摩尔水平的强效 HDAC6 抑制作用。几种化合物表现出显着的 HDAC6 抑制活性(IC 50 = 48.85–75.62 nM),优于参考药物辛二酰苯胺异羟肟酸(SAHA;IC 50 = 91.73 纳米)。进一步评估了最有效的衍生物对两种人类白血病细胞系的体外抗癌活性。硫代巴比妥酸盐3e对测试细胞的效力是 SAHA 的两倍。还描述了详细的构效关系。此外,分子对接模拟揭示了标题化合物螯合位于 HDAC6 结合口袋内的催化 Zn +2离子的能力。物理化学性质的计算机评价表明,目标化合物在药代动力学方面是有前途的候选者。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号