当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Batch and continuous flow asymmetric synthesis of anabolic-androgenic steroids via a single-cell biocatalytic Δ1-dehydrogenation and C17β-carbonyl reduction cascade
Green Chemistry ( IF 9.3 ) Pub Date : 2023-03-21 , DOI: 10.1039/d2gc04894a Yajiao Zhang 1 , Minjie Liu 1 , Zixin Yang 2 , Juan Lin 1 , Zedu Huang 3, 4 , Fener Chen 1, 2, 3, 4
Green Chemistry ( IF 9.3 ) Pub Date : 2023-03-21 , DOI: 10.1039/d2gc04894a Yajiao Zhang 1 , Minjie Liu 1 , Zixin Yang 2 , Juan Lin 1 , Zedu Huang 3, 4 , Fener Chen 1, 2, 3, 4
Affiliation
|
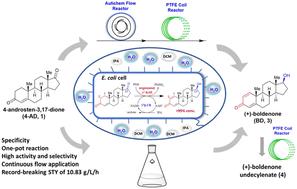
Chemoenzymatic asymmetric synthesis of an anabolic-androgenic steroid (+)-boldenone (3) and its prodrug (+)-boldenone undecylenate (4) was accomplished starting from commercially available 4-androstene-3,17-dione (4-AD, 1) under both batch and continuous flow conditions. The key feature of the current synthesis is the construction of an enzymatic cascade process in a single Escherichia coli cell for straightforward synthesis of (+)-boldenone (3), enabled by the combined action of ReM2 (I51L/I350T), an engineered 3-ketosteroid-Δ1-dehydrogenase (Δ1-KstD) possessing 5-fold and 3-fold higher Δ1-dehydrogenation activity towards 4-AD and testosterone (2b) relative to the wild-type Δ1-KstD, respectively, and 17β-CR, a newly mined carbonyl reductase from Empedobacter stercoris showing strong C17-carbonyl reduction activity. With the optimal reaction conditions established for mutual tolerance between ReM2 and 17β-CR, complete conversion of 4-AD into (+)-boldenone was first realized in a conventional batch mode with a space-time yield (STY) of 1.09 g L−1 h−1. Furthermore, this single cell-catalyzed synthesis of (+)-boldenone was successfully implemented in continuous flow, achieving an order of magnitude higher STY (10.83 g L−1 h−1) than that for batch synthesis, which also represents the highest record for the biocatalytic synthesis of (+)-boldenone reported to date. Finally, (+)-boldenone undecylenate (4) was produced in a fully continuous flow mode with an overall yield of 75%, through telescoping the newly developed biocatalytic Δ1-dehydrogenation/17β-carbonyl reduction cascade with the follow-up esterification reaction. The present work not only provides a concise, efficient, and sustainable avenue for the asymmetric synthesis of (+)-boldenone and (+)-boldenone undecylenate, but also showcases the effectiveness and great potential of flow biocatalysis in the production of value-added compounds.
中文翻译:

通过单细胞生物催化 Δ1-脱氢和 C17β-羰基还原级联合成代谢雄激素的批量和连续流不对称合成
Chemoenzymatic asymmetric synthesis of an anabolic-androgenic steroid (+)-boldenone (3) and its prodrug (+)-boldenone undecylenate (4) was accomplished starting from commercially available 4-androstene-3,17-dione (4-AD, 1) under both batch and continuous flow conditions. The key feature of the current synthesis is the construction of an enzymatic cascade process in a single Escherichia coli cell for straightforward synthesis of (+)-boldenone (3), enabled by the combined action of ReM2 (I51L/I350T), an engineered 3-ketosteroid-Δ1-dehydrogenase (Δ1-KstD) possessing 5-fold and 3-fold higher Δ1- 相对于野生型 Δ 1 -KstD 和 17β-CR 的4-AD 和睾酮 ( 2b ) 的脱氢活性,17β-CR 是一种新发现的来自粪肠杆菌的羰基还原酶,显示出很强的 C17-羰基还原活性。通过为 ReM2 和 17β-CR 之间的相互耐受性建立最佳反应条件,4-AD 完全转化为 (+)-boldenone 首次以传统的分批模式实现,时空产率 (STY) 为 1.09 g L - 1小时-1。此外,这种单细胞催化的 (+)-boldenone 合成在连续流中成功实施,实现了一个数量级的高 STY(10.83 g L −1 h −1) 高于批量合成,这也代表了迄今为止报道的 (+)-boldenone 生物催化合成的最高记录。最后,通过将新开发的生物催化 Δ 1 -脱氢/17β-羰基还原级联与后续酯化反应进行伸缩,以完全连续流动模式生产 (+)-boldenone undecylenate ( 4 ),总收率为 75% . 目前的工作不仅为 (+)-boldenone 和 (+)-boldenone undecylenate 的不对称合成提供了简洁、高效和可持续的途径,而且展示了流动生物催化在生产增值产品中的有效性和巨大潜力化合物。
更新日期:2023-03-21
中文翻译:

通过单细胞生物催化 Δ1-脱氢和 C17β-羰基还原级联合成代谢雄激素的批量和连续流不对称合成
Chemoenzymatic asymmetric synthesis of an anabolic-androgenic steroid (+)-boldenone (3) and its prodrug (+)-boldenone undecylenate (4) was accomplished starting from commercially available 4-androstene-3,17-dione (4-AD, 1) under both batch and continuous flow conditions. The key feature of the current synthesis is the construction of an enzymatic cascade process in a single Escherichia coli cell for straightforward synthesis of (+)-boldenone (3), enabled by the combined action of ReM2 (I51L/I350T), an engineered 3-ketosteroid-Δ1-dehydrogenase (Δ1-KstD) possessing 5-fold and 3-fold higher Δ1- 相对于野生型 Δ 1 -KstD 和 17β-CR 的4-AD 和睾酮 ( 2b ) 的脱氢活性,17β-CR 是一种新发现的来自粪肠杆菌的羰基还原酶,显示出很强的 C17-羰基还原活性。通过为 ReM2 和 17β-CR 之间的相互耐受性建立最佳反应条件,4-AD 完全转化为 (+)-boldenone 首次以传统的分批模式实现,时空产率 (STY) 为 1.09 g L - 1小时-1。此外,这种单细胞催化的 (+)-boldenone 合成在连续流中成功实施,实现了一个数量级的高 STY(10.83 g L −1 h −1) 高于批量合成,这也代表了迄今为止报道的 (+)-boldenone 生物催化合成的最高记录。最后,通过将新开发的生物催化 Δ 1 -脱氢/17β-羰基还原级联与后续酯化反应进行伸缩,以完全连续流动模式生产 (+)-boldenone undecylenate ( 4 ),总收率为 75% . 目前的工作不仅为 (+)-boldenone 和 (+)-boldenone undecylenate 的不对称合成提供了简洁、高效和可持续的途径,而且展示了流动生物催化在生产增值产品中的有效性和巨大潜力化合物。































 京公网安备 11010802027423号
京公网安备 11010802027423号