当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, fabrication and characterization of 2-naphthyloxy group-substituted bis(2-pyridylimino)isoindoline and its derivatives as a positive electrode for vanadium redox flow battery applications
Dalton Transactions ( IF 3.5 ) Pub Date : 2023-03-18 , DOI: 10.1039/d2dt03547b Selin Gümrükçü 1 , Mukaddes Özçeşmeci 1 , Nilüfer Koçyiğit 2 , Kerem Kaya 1 , Ahmet Gül 1 , Yücel Şahin 3 , İbrahim Özçeşmeci 1
Dalton Transactions ( IF 3.5 ) Pub Date : 2023-03-18 , DOI: 10.1039/d2dt03547b Selin Gümrükçü 1 , Mukaddes Özçeşmeci 1 , Nilüfer Koçyiğit 2 , Kerem Kaya 1 , Ahmet Gül 1 , Yücel Şahin 3 , İbrahim Özçeşmeci 1
Affiliation
|
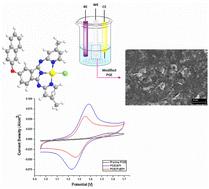
In recent years, tridentate nitrogen donor ligands have played a vital role in inorganic chemistry. The ease of synthesis, readily modifiable structure and high stability of 1,3-bis(2-pyridylimino)isoindole (BPIs) compounds make them suitable candidates for many potential applications. In this study, a 1,3-bis(2-pyridylimino)isoindoline derivative bearing a naphthoxy unit and its palladium complex (PdBPI) were synthesized and characterized by single crystal X-ray diffraction, NMR, FT-IR, UV–Vis, and mass spectroscopic methods. The BPI- or PdBPI-modified pencil graphite electrodes were clarified via cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), scanning electron microscopy (SEM), EDX, X-ray photoelectron spectroscopy (XPS) and Raman spectroscopy. The efficiency of these substances in a vanadium redox flow battery (VRB) system was investigated for the first time. The behaviors of the BPI-modified carbon felt electrode (BPI-CF) and PdBPI-modified carbon felt electrode (PdBPI-CF) were investigated in the redox flow battery (RFB) applications. These modified electrodes were obtained by the electrodeposition method. The respective charge potentials of BPI-CF and PdBPI-CF reached 1.63 V and 1.88 V, respectively. The discharge capacity maxima obtained were ∼301 mA h (1204 mA h L−1) and ∼303 mA h (1212 mA h L−1) for BPI-CF and PdBPI-CF at the VRB system under a charge current density of 4.0 mA cm−2 and discharge current density of 0.4 mA cm−2, respectively.
中文翻译:

2-萘氧基取代的双(2-吡啶亚氨基)异二氢吲哚及其衍生物的合成、制备和表征作为钒氧化还原液流电池应用的正极
近年来,三齿氮供体配体在无机化学中发挥了重要作用。1,3-双(2-吡啶亚氨基)异吲哚 (BPI) 化合物易于合成、易于修饰的结构和高稳定性使其成为许多潜在应用的合适候选者。在这项研究中,合成了一种带有萘氧基单元的 1,3-双(2-吡啶亚氨基)异二氢吲哚衍生物及其钯络合物 (PdBPI),并通过单晶 X 射线衍射、NMR、FT-IR、UV-Vis、和质谱方法。BPI 或 PdBPI 改性的铅笔石墨电极通过循环伏安法 (CV)、电化学阻抗谱 (EIS)、扫描电子显微镜 (SEM)、EDX、X 射线光电子能谱 (XPS) 和拉曼光谱。首次研究了这些物质在钒氧化还原液流电池 (VRB) 系统中的效率。在氧化还原液流电池 (RFB) 应用中研究了 BPI 改性碳毡电极 (BPI-CF) 和 PdBPI 改性碳毡电极 (PdBPI-CF) 的行为。这些修饰电极是通过电沉积法获得的。BPI-CF和PdBPI-CF各自的电荷电位分别达到1.63 V和1.88 V。获得的放电容量最大值为~301 mA h (1204 mA h L -1 )和~303 mA h (1212 mA h L -1) 对于 VRB 系统中的 BPI-CF 和 PdBPI-CF,充电电流密度分别为 4.0 mA cm -2和放电电流密度为 0.4 mA cm -2。
更新日期:2023-03-18
中文翻译:

2-萘氧基取代的双(2-吡啶亚氨基)异二氢吲哚及其衍生物的合成、制备和表征作为钒氧化还原液流电池应用的正极
近年来,三齿氮供体配体在无机化学中发挥了重要作用。1,3-双(2-吡啶亚氨基)异吲哚 (BPI) 化合物易于合成、易于修饰的结构和高稳定性使其成为许多潜在应用的合适候选者。在这项研究中,合成了一种带有萘氧基单元的 1,3-双(2-吡啶亚氨基)异二氢吲哚衍生物及其钯络合物 (PdBPI),并通过单晶 X 射线衍射、NMR、FT-IR、UV-Vis、和质谱方法。BPI 或 PdBPI 改性的铅笔石墨电极通过循环伏安法 (CV)、电化学阻抗谱 (EIS)、扫描电子显微镜 (SEM)、EDX、X 射线光电子能谱 (XPS) 和拉曼光谱。首次研究了这些物质在钒氧化还原液流电池 (VRB) 系统中的效率。在氧化还原液流电池 (RFB) 应用中研究了 BPI 改性碳毡电极 (BPI-CF) 和 PdBPI 改性碳毡电极 (PdBPI-CF) 的行为。这些修饰电极是通过电沉积法获得的。BPI-CF和PdBPI-CF各自的电荷电位分别达到1.63 V和1.88 V。获得的放电容量最大值为~301 mA h (1204 mA h L -1 )和~303 mA h (1212 mA h L -1) 对于 VRB 系统中的 BPI-CF 和 PdBPI-CF,充电电流密度分别为 4.0 mA cm -2和放电电流密度为 0.4 mA cm -2。











































 京公网安备 11010802027423号
京公网安备 11010802027423号