Green Synthesis and Catalysis ( IF 8.2 ) Pub Date : 2022-07-03 , DOI: 10.1016/j.gresc.2022.06.010 Yunfeng Cui , Yangyang Ji , Xi Chen , Jianjiong Li , Jinhui Feng , Qing Zhao , Peiyuan Yao , Qiaqing Wu , Dunming Zhu
|
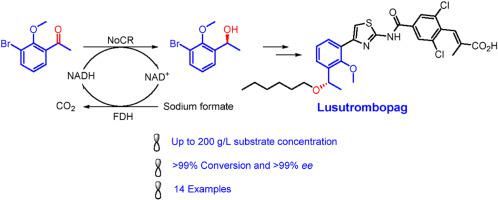
(S)-1-(3′-Bromo-2′-methoxyphenyl)ethanol ((S)-1b) is the key precursor for the synthesis of Lusutrombopag. The bioreduction of 1-(3′-bromo-2′-methoxyphenyl)ethanone (1a) offers an attractive method to access this important compound. Through screening the available carbonyl reductases, we obtained a carbonyl reductase from Novosphingobium aromaticivorans (CBR), which could completely convert 100 g/L of 1a to (S)-1b. Furthermore, a carbonyl reductase from Novosphingobium sp. Leaf2 (NoCR) was identified to completely convert 200 g/L of 1a to (S)-1b with excellent enantioselectivity (>99% ee) and 77% isolated yield using FDH/formate system for NADH regeneration. The Km and kcat of recombinant NoCR towards 1a were 0.66 mmol/L and 7.5 s-1, and the catalytic efficiency kcat/Km was 11.3 mmol/s.L. Meanwhile, NoCR showed high catalytic activity and stereoselectivity towards acetophenone derivatives with halogen or methoxy substitution on the benzene ring, indicating that NoCR is a valuable biocatalyst with potential practical applications.
中文翻译:

高效酶法合成 (S)-1-(3′-溴-2′-甲氧基苯基)乙醇(lusutrombopag 的关键组成部分)
( S )-1-(3′-溴-2′-甲氧基苯基)乙醇(( S ) -1b )是合成Lusutrombopag的关键前体。1-(3'-溴-2'-甲氧基苯基)乙酮 ( 1a ) 的生物还原为获取这种重要化合物提供了一种有吸引力的方法。通过筛选可用的羰基还原酶,我们获得了一种来自Novosphingobiumaromaticivorans(CBR)的羰基还原酶,它可以将100g/L的1a完全转化为(S)-1b。此外,来自Novosphingobium sp的羰基还原酶。Leaf2 (NoCR) 经鉴定可将 200 g/L 的1a完全转化为 ( S )-1b使用 FDH/甲酸盐系统进行 NADH 再生时具有出色的对映选择性 (>99% ee ) 和 77% 的分离产率。重组NoCR对1a的K m和k cat分别为0.66 mmol/L和7.5 s -1 ,催化效率k cat /K m为11.3 mmol/s 。同时,NoCR对苯环上有卤素或甲氧基取代的苯乙酮衍生物表现出较高的催化活性和立体选择性,表明NoCR是一种有价值的生物催化剂,具有潜在的实际应用价值。































 京公网安备 11010802027423号
京公网安备 11010802027423号