Structure ( IF 4.4 ) Pub Date : 2023-03-16 , DOI: 10.1016/j.str.2023.02.009
Prabhanshu Tripathi 1 , Michael F Bender 1 , Haotian Lei 2 , Lais Da Silva Pereira 1 , Chen-Hsiang Shen 1 , Brian Bonilla 1 , Marlon Dillon 1 , Li Ou 1 , Marie Pancera 3 , Lawrence T Wang 1 , Baoshan Zhang 1 , Facundo D Batista 4 , Azza H Idris 1 , Robert A Seder 1 , Peter D Kwong 1
|
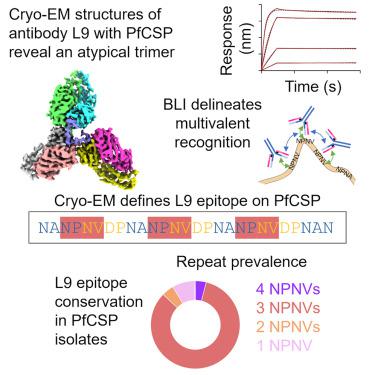
Monoclonal antibody L9 recognizes the Plasmodium falciparum circumsporozoite protein (PfCSP) and is highly protective following controlled human malaria challenge. To gain insight into its function, we determined cryoelectron microscopy (cryo-EM) structures of L9 in complex with full-length PfCSP and assessed how this recognition influenced protection by wild-type and mutant L9s. Cryo-EM reconstructions at 3.6- and 3.7-Å resolution revealed L9 to recognize PfCSP as an atypical trimer. Each of the three L9s in the trimer directly recognized an Asn-Pro-Asn-Val (NPNV) tetrapeptide on PfCSP and interacted homotypically to facilitate L9-trimer assembly. We analyzed peptides containing different repeat tetrapeptides for binding to wild-type and mutant L9s to delineate epitope and homotypic components of L9 recognition; we found both components necessary for potent malaria protection. Last, we found the 27-residue stretch recognized by L9 to be highly conserved in P. falciparum isolates, suggesting the newly revealed complete L9 epitope to be an attractive vaccine target.
中文翻译:

抗疟疾抗体 L9 与环子孢子蛋白的冷冻电镜结构揭示了三聚体 L9 关联和完整的 27 个残基表位
单克隆抗体 L9 可识别恶性疟原虫环子孢子蛋白 (PfCSP),并且在受控的人类疟疾攻击后具有高度保护性。为了深入了解其功能,我们确定了 L9 与全长 PfCSP 复合物的冷冻电子显微镜 (cryo-EM) 结构,并评估了这种识别如何影响野生型和突变型 L9 的保护作用。 3.6 和 3.7 Å 分辨率的冷冻电镜重建显示 L9 将 PfCSP 识别为非典型三聚体。三聚体中的三个 L9 均直接识别 PfCSP 上的 Asn-Pro-Asn-Val (NPNV) 四肽,并同型相互作用以促进 L9 三聚体组装。我们分析了含有不同重复四肽的肽与野生型和突变型 L9 的结合,以描绘 L9 识别的表位和同型成分;我们发现这两种成分都是有效预防疟疾所必需的。最后,我们发现 L9 识别的 27 个残基序列在恶性疟原虫分离株中高度保守,这表明新发现的完整 L9 表位是一个有吸引力的疫苗靶点。

































 京公网安备 11010802027423号
京公网安备 11010802027423号