Molecular Therapy ( IF 12.1 ) Pub Date : 2023-03-16 , DOI: 10.1016/j.ymthe.2023.03.011
Kelly Hanna 1 , Julio Nieves 1 , Christine Dowd 1 , Kristina Oresic Bender 1 , Pallavi Sharma 1 , Baljit Singh 1 , Mark Renz 1 , James N Ver Hoeve 2 , Diana Cepeda 1 , Claire M Gelfman 1 , Brigit E Riley 1 , Ruslan N Grishanin 1
|
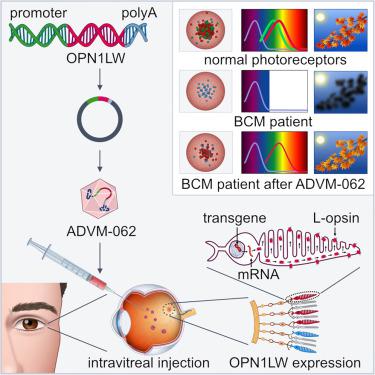
Blue cone monochromacy (BCM) is a rare X-linked retinal disease characterized by the absence of L- and M-opsin in cone photoreceptors, considered a potential gene therapy candidate. However, most experimental ocular gene therapies utilize subretinal vector injection which would pose a risk to the fragile central retinal structure of BCM patients. Here we describe the use of ADVM-062, a vector optimized for cone-specific expression of human L-opsin and administered using a single intravitreal (IVT) injection. Pharmacological activity of ADVM-062 was established in gerbils, whose cone-rich retina naturally lacks L-opsin. A single IVT administration dose of ADVM-062 effectively transduced gerbil cone photoreceptors and produced a de novo response to long-wavelength stimuli. To identify potential first-in-human doses we evaluated ADVM-062 in non-human primates. Cone-specific expression of ADVM-062 in primates was confirmed using ADVM-062.myc, a vector engineered with the same regulatory elements as ADVM-062. Enumeration of human OPN1LW.myc-positive cones demonstrated that doses ≥3 × 1010 vg/eye resulted in transduction of 18%–85% of foveal cones. A Good Laboratory Practice (GLP) toxicology study established that IVT administration of ADVM-062 was well tolerated at doses that could potentially achieve clinically meaningful effect, thus supporting the potential of ADVM-062 as a one-time IVT gene therapy for BCM.
中文翻译:

ADVM-062(一种用于治疗蓝锥体单色性的新型玻璃体内基因治疗载体)的临床前评估
蓝视锥单色性 (BCM) 是一种罕见的 X 连锁视网膜疾病,其特征是视锥光感受器中缺乏 L-和 M-视蛋白,被认为是潜在的基因治疗候选者。然而,大多数实验性眼部基因疗法采用视网膜下载体注射,这会对 BCM 患者脆弱的中央视网膜结构构成风险。在这里,我们描述了 ADVM-062 的使用,这是一种针对人 L-视蛋白视锥特异性表达进行优化的载体,并通过单次玻璃体内 (IVT) 注射进行给药。 ADVM-062 的药理活性是在沙鼠身上建立的,沙鼠的视锥细胞丰富的视网膜天然缺乏 L-视蛋白。 ADVM-062 的单次 IVT 给药剂量可有效转导沙鼠视锥光感受器,并对长波长刺激产生从头反应。为了确定潜在的首次人体剂量,我们在非人类灵长类动物中评估了 ADVM-062。使用 ADVM-062.myc 证实了 ADVM-062 在灵长类动物中的视锥细胞特异性表达,ADVM-062.myc 是一种采用与 ADVM-062 相同调控元件设计的载体。对人类 OPN1LW.myc 阳性视锥细胞的计数表明,≥3 × 10 10 vg/眼的剂量导致 18%–85% 的中央凹视锥细胞转导。一项良好实验室规范 (GLP) 毒理学研究表明,ADVM-062 的 IVT 给药剂量具有良好的耐受性,可能会实现具有临床意义的效果,从而支持 ADVM-062 作为 BCM 一次性 IVT 基因疗法的潜力。


































 京公网安备 11010802027423号
京公网安备 11010802027423号