当前位置:
X-MOL 学术
›
Carbon Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Balancing sub-reaction activity to boost electrocatalytic urea synthesis using a metal-free electrocatalyst
Carbon Energy ( IF 19.5 ) Pub Date : 2023-03-17 , DOI: 10.1002/cey2.345 Chen Chen 1 , Shuang Li 1 , Xiaorong Zhu 2 , Shuowen Bo 3 , Kai Cheng 4 , Nihan He 1 , Mengyi Qiu 1 , Chao Xie 1 , Dezhong Song 1 , Youzhen Liu 1 , Wei Chen 1 , Yafei Li 2 , Qinghua Liu 3 , Conggang Li 4 , Shuangyin Wang 1
Carbon Energy ( IF 19.5 ) Pub Date : 2023-03-17 , DOI: 10.1002/cey2.345 Chen Chen 1 , Shuang Li 1 , Xiaorong Zhu 2 , Shuowen Bo 3 , Kai Cheng 4 , Nihan He 1 , Mengyi Qiu 1 , Chao Xie 1 , Dezhong Song 1 , Youzhen Liu 1 , Wei Chen 1 , Yafei Li 2 , Qinghua Liu 3 , Conggang Li 4 , Shuangyin Wang 1
Affiliation
|
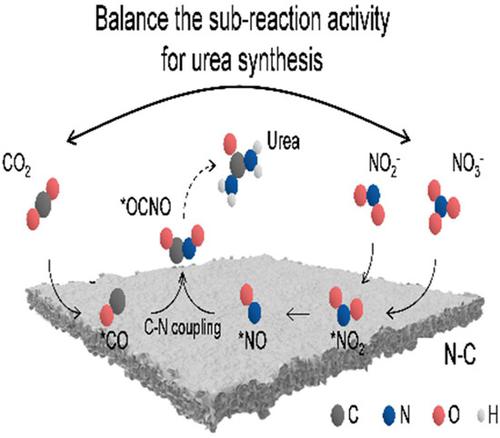
Electrocatalytic urea synthesis via coupling of nitrate with CO2 is considered as a promising alternative to the industrial urea synthetic process. However, the requirement of sub-reaction (NO3RR and CO2RR) activities for efficient urea synthesis is not clear and the related reaction mechanisms remain obscure. Here, the construction, breaking, and rebuilding of the sub-reaction activity balance would be accompanied by the corresponding regulation in urea synthesis, and the balance of sub-reaction activities was proven to play a vital role in efficient urea synthesis. With rational design, a urea yield rate of 610.6 mg h−1 gcat.−1 was realized on the N-doped carbon electrocatalyst, superior to that of noble-metal electrocatalysts. Based on the operando SR-FTIR measurements, we proposed that urea synthesis arises from the coupling of *NO and *CO to generate the key intermediate of *OCNO. This work provides new insights and guidelines into urea synthesis from the aspect of activity balance.
中文翻译:

使用无金属电催化剂平衡子反应活性以促进电催化尿素合成
通过硝酸盐与CO 2偶联的电催化尿素合成被认为是工业尿素合成工艺的有前途的替代方案。然而,有效合成尿素的子反应(NO 3 RR和CO 2 RR)活性的要求尚不清楚,相关反应机制仍不清楚。这里,子反应活性平衡的构建、打破和重建将伴随着尿素合成中的相应调节,并且子反应活性的平衡被证明在尿素高效合成中发挥着至关重要的作用。通过合理设计,尿素收率为610.6 mg h -1 g cat。-1在N掺杂碳电催化剂上实现,优于贵金属电催化剂。基于操作 SR-FTIR 测量,我们提出尿素合成是由 *NO 和 *CO 偶联产生 *OCNO 的关键中间体。这项工作从活性平衡的角度为尿素合成提供了新的见解和指导。
更新日期:2023-03-17
中文翻译:

使用无金属电催化剂平衡子反应活性以促进电催化尿素合成
通过硝酸盐与CO 2偶联的电催化尿素合成被认为是工业尿素合成工艺的有前途的替代方案。然而,有效合成尿素的子反应(NO 3 RR和CO 2 RR)活性的要求尚不清楚,相关反应机制仍不清楚。这里,子反应活性平衡的构建、打破和重建将伴随着尿素合成中的相应调节,并且子反应活性的平衡被证明在尿素高效合成中发挥着至关重要的作用。通过合理设计,尿素收率为610.6 mg h -1 g cat。-1在N掺杂碳电催化剂上实现,优于贵金属电催化剂。基于操作 SR-FTIR 测量,我们提出尿素合成是由 *NO 和 *CO 偶联产生 *OCNO 的关键中间体。这项工作从活性平衡的角度为尿素合成提供了新的见解和指导。






























 京公网安备 11010802027423号
京公网安备 11010802027423号