当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface Processes Control the Fate of Reactive Oxidants Generated by Electrochemical Activation of Hydrogen Peroxide on Stainless-Steel Electrodes
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-03-16 , DOI: 10.1021/acs.est.2c08404 Yanghua Duan 1 , Wenli Jiang 1 , David L Sedlak 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-03-16 , DOI: 10.1021/acs.est.2c08404 Yanghua Duan 1 , Wenli Jiang 1 , David L Sedlak 1
Affiliation
|
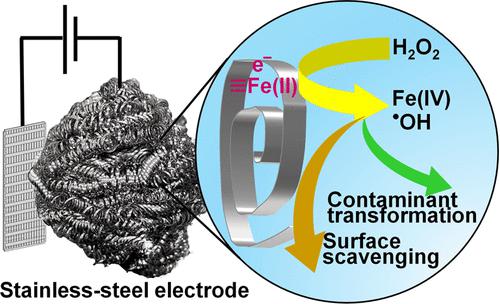
Low-cost stainless-steel electrodes can activate hydrogen peroxide (H2O2) by converting it into a hydroxyl radical (•OH) and other reactive oxidants. At an applied potential of +0.020 V, the stainless-steel electrode produced •OH with a yield that was over an order of magnitude higher than that reported for other systems that employ iron oxides as catalysts under circumneutral pH conditions. Decreasing the applied potential at pH 8 and 9 enhanced the rate of H2O2 loss by shifting the process to a reaction mechanism that resulted in the formation of an Fe(IV) species. Significant metal leaching was only observed under acidic pH conditions (i.e., at pH <6), with the release of dissolved Fe and Cr occurring as the thickness of the passivation layer decreased. Despite the relatively high yield of •OH production under circumneutral pH conditions, most of the oxidants were scavenged by the electrode surface when contaminant concentrations comparable to those expected in drinking water sources were tested. The stainless-steel electrode efficiently removed trace organic contaminants from an authentic surface water sample without contaminating the water with Fe and Cr. With further development, stainless-steel electrodes could provide a cost-effective alternative to other H2O2 activation processes, such as those by ultraviolet light.
中文翻译:

表面处理控制不锈钢电极上过氧化氢电化学活化产生的活性氧化剂的命运
低成本的不锈钢电极可以通过将过氧化氢(H 2 O 2 )转化为羟基自由基( · OH)和其他活性氧化剂来激活它。在+0.020 V 的施加电位下,不锈钢电极产生• OH,其产率比在中性pH 条件下采用氧化铁作为催化剂的其他系统的产率高出一个数量级以上。在 pH 8 和 9 下降低施加的电位可通过将过程转变为导致 Fe(IV) 物质形成的反应机制来提高 H 2 O 2损失速率。仅在酸性 pH 条件下观察到显着的金属浸出(即,pH <6 id=8>• 在中性 pH 条件下产生 OH,当污染物浓度与饮用水源中预期的浓度相当时,大多数氧化剂被电极表面清除经测试,不锈钢电极可有效去除真实地表水样中的微量有机污染物,且不会被铁和铬污染。随着进一步的发展,不锈钢电极可以提供一种经济有效的替代方法来替代其他 H 2 O 2活化方法。过程,例如通过紫外线进行的过程。
更新日期:2023-03-16
中文翻译:

表面处理控制不锈钢电极上过氧化氢电化学活化产生的活性氧化剂的命运
低成本的不锈钢电极可以通过将过氧化氢(H 2 O 2 )转化为羟基自由基( · OH)和其他活性氧化剂来激活它。在+0.020 V 的施加电位下,不锈钢电极产生• OH,其产率比在中性pH 条件下采用氧化铁作为催化剂的其他系统的产率高出一个数量级以上。在 pH 8 和 9 下降低施加的电位可通过将过程转变为导致 Fe(IV) 物质形成的反应机制来提高 H 2 O 2损失速率。仅在酸性 pH 条件下观察到显着的金属浸出(即,pH <6 id=8>• 在中性 pH 条件下产生 OH,当污染物浓度与饮用水源中预期的浓度相当时,大多数氧化剂被电极表面清除经测试,不锈钢电极可有效去除真实地表水样中的微量有机污染物,且不会被铁和铬污染。随着进一步的发展,不锈钢电极可以提供一种经济有效的替代方法来替代其他 H 2 O 2活化方法。过程,例如通过紫外线进行的过程。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号