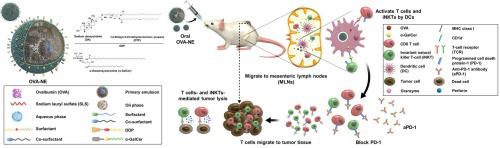
我们开发了一种可诱导癌症免疫的口服纳米乳剂。它由载有有效不变自然杀伤 T 细胞 (iNKT) 激活剂 α-半乳糖苷神经酰胺 (α-GalCer) 的载有肿瘤抗原的纳米囊泡组成,可通过有效激活先天免疫和适应性免疫来触发癌症免疫。经证实,向系统中添加胆汁盐可促进肠道淋巴转运以及卵清蛋白 (OVA) 的口服生物利用度乳糜微粒途径。为了进一步增加肠道通透性并增强抗肿瘤反应,将阳离子脂质 1,2-二油基-3-三甲基丙烷 (DTP) 与脱氧胆酸钠 (DA) (DDP) 和 α-GalCer 的离子复合物锚定在外油层上形成 OVA-NE#3。正如预期的那样,OVA-NE#3 表现出极大改善的肠细胞通透性以及增强的向肠系膜淋巴结 (MLN) 的递送。还观察到 MLN 中树突状细胞和 iNKT 的后续激活。与未治疗的对照组相比,口服 OVA-NE#3 后,表达 OVA 的黑素瘤小鼠的肿瘤生长受到更强烈的抑制(71%),证实了该系统诱导的强烈免疫反应。OVA 特异性 IgG1 和 IgG2a 的血清水平比对照组高 3.52 倍和 6.14 倍。处理 OVA-NE#3 会增加肿瘤浸润淋巴细胞的数量,包括细胞毒性 T 细胞和 M1 样巨噬细胞。OVA-NE#3 处理后,肿瘤组织中抗原和 α-GalCer 相关的树突状细胞和 iNKT 富集也增加。这些观察结果表明我们的系统通过靶向口腔淋巴系统诱导细胞免疫和体液免疫。它可能提供一种有前途的口服抗癌疫苗接种策略,包括诱导全身抗癌免疫。这些观察结果表明我们的系统通过靶向口腔淋巴系统诱导细胞免疫和体液免疫。它可能提供一种有前途的口服抗癌疫苗接种策略,包括诱导全身抗癌免疫。这些观察结果表明我们的系统通过靶向口腔淋巴系统诱导细胞免疫和体液免疫。它可能提供一种有前途的口服抗癌疫苗接种策略,包括诱导全身抗癌免疫。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Oral lymphatic delivery of alpha-galactosylceramide and ovalbumin evokes anti-cancer immunization
We developed an orally delivered nanoemulsion that induces cancer immunization. It consists of tumor antigen-loaded nano-vesicles carrying the potent invariant natural killer T-cell (iNKT) activator α-galactosylceramide (α-GalCer), to trigger cancer immunity by effectively activating both innate and adaptive immunity. It was validated that adding bile salts to the system boosted intestinal lymphatic transport as well as the oral bioavailability of ovalbumin (OVA) via the chylomicron pathway. To increase intestinal permeability further and amplify the antitumor responses, an ionic complex of cationic lipid 1,2-dioleyl-3-trimethylammonium propane (DTP) with sodium deoxycholate (DA) (DDP) and α-GalCer were anchored onto the outer oil layer to form OVA-NE#3. As expected, OVA-NE#3 exhibited tremendously improved intestinal cell permeability as well as enhanced delivery to mesenteric lymph nodes (MLNs). Subsequent activation of dendritic cells and iNKTs, in MLNs were also observed. Tumor growth in OVA-expressing mice with melanoma was more strongly suppressed (by 71%) after oral administration of OVA-NE#3 than in untreated controls, confirming the strong immune response induced by the system. The serum levels of OVA-specific IgG1 and IgG2a were 3.52- and 6.14-fold higher than in controls. Treating OVA-NE#3 increased the numbers of tumor-infiltrating lymphocytes, including cytotoxic T-cell and M1-like macrophage. Antigen- and α-GalCer-associated enrichment of dendritic cells and iNKTs in tumor tissues also increased after OVA-NE#3 treatment. These observations indicate that our system induces both cellular and humoral immunity by targeting the oral lymphatic system. It may offer a promising oral anti-cancer vaccination strategy that involves the induction of systemic anti-cancer immunization.



















































 京公网安备 11010802027423号
京公网安备 11010802027423号