当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isotopic Constraints on the Nature of Primary Precipitates in Archean–Early Paleoproterozoic Iron Formations from Determinations of the Iron Phonon Density of States of Greenalite and 2L- and 6L-Ferrihydrite
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2023-03-15 , DOI: 10.1021/acsearthspacechem.2c00313 Andy W. Heard 1, 2, 3 , Nicolas Dauphas 1 , Isaac L. Hinz 4 , Jena E. Johnson 4 , Marc Blanchard 5 , Esen E. Alp 1 , Michael Y. Hu 1 , Jiyong Zhao 1 , Barbara Lavina 1, 6 , Mark E. Fornace 1, 7 , Justin Y. Hu 1, 8 , Mathieu Roskosz 9 , Corliss Kin I Sio 10 , Nicole X. Nie 11 , Benoît Baptiste 9
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2023-03-15 , DOI: 10.1021/acsearthspacechem.2c00313 Andy W. Heard 1, 2, 3 , Nicolas Dauphas 1 , Isaac L. Hinz 4 , Jena E. Johnson 4 , Marc Blanchard 5 , Esen E. Alp 1 , Michael Y. Hu 1 , Jiyong Zhao 1 , Barbara Lavina 1, 6 , Mark E. Fornace 1, 7 , Justin Y. Hu 1, 8 , Mathieu Roskosz 9 , Corliss Kin I Sio 10 , Nicole X. Nie 11 , Benoît Baptiste 9
Affiliation
|
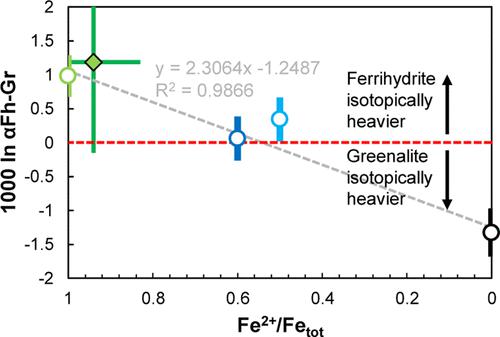
Iron formations (IFs) are chemical sedimentary rocks that were widely deposited before the Great Oxidation Event (GOE) around 2.4–2.2 Ga. It is generally thought that IFs precipitated as hydrated Fe3+ oxides (HFOs) such as ferrihydrite following surface oxidation of Fe2+-rich, anoxic deep waters. This model often implicates biological oxidation and underpins reconstructions of marine nutrient concentrations. However, nanoscale petrography indicates that an Fe2+ silicate, greenalite, is a common primary mineral in well-preserved IFs, motivating an alternative depositional model of anoxic ferrous silicate precipitation. It is unclear, however, if Fe2+-rich silicates can produce the Fe isotopic variations in IFs that are well explained by Fe2+ oxidation. To address this question, we constrain the equilibrium Fe isotopic (56Fe/54Fe) fractionation of greenalite and ferrihydrite by determining the iron phonon densities of states for those minerals. We use ab initio density functional theory (DFT + U) calculations and nuclear resonant inelastic X-ray scattering spectroscopy to show that ferrous greenalite should be isotopically lighter than ferrihydrite by ∼1–1.2‰ at equilibrium, and fractionation should scale linearly with increasing Fe3+ content in greenalite. By anchoring ferrihydrite–greenalite mineral pair fractionations to published experimental Fe isotopic fractionations between HFOs and aqueous Fe2+, we show that ferrous greenalite may produce all but the heaviest pre-GOE Fe isotopic compositions and mixed valence greenalites can produce the entire record. Our results suggest that heavy Fe isotope enrichments alone are not diagnostic of primary IF mineralogies, and ferrihydrite and partially oxidized or even purely ferrous greenalite are all viable primary IF mineralogies.
中文翻译:

太古代-早古元古代铁地层中初级沉淀物性质的同位素限制,来自绿铝石和 2L- 和 6L- 水铁矿状态的铁声子密度的测定
铁地层 (IFs) 是在大氧化事件 (GOE) 之前广泛沉积的化学沉积岩,大约 2.4–2.2 Ga。通常认为 IFs 在表面氧化后沉淀为水合 Fe 3+ 氧化物 (HFO),例如水铁矿富含Fe 2+的缺氧深水。该模型通常涉及生物氧化并支持海洋营养物浓度的重建。然而,纳米岩相学表明,Fe 2+硅酸盐绿岩是保存完好的 IFs 中常见的原生矿物,激发了缺氧硅酸亚铁沉淀的替代沉积模型。然而,尚不清楚 Fe 2+- 富含硅酸盐可以在 IF 中产生 Fe 同位素变化,这可以通过 Fe 2+氧化得到很好的解释。为了解决这个问题,我们通过确定这些矿物的铁声子态密度来限制绿铝石和水铁矿的平衡Fe 同位素 ( 56 Fe/ 54 Fe)分馏。我们使用从头算密度泛函理论 (DFT + U ) 计算和核共振非弹性 X 射线散射光谱表明,在平衡状态下,亚铁绿铁矿在同位素方面应比水铁矿轻 ~1–1.2‰,并且分馏应随 Fe 的增加呈线性变化3+绿岩中的含量。通过将水铁矿-绿铝石矿物对分馏锚定到已发表的 HFO 和 Fe 2+水溶液之间的实验性 Fe 同位素分馏,我们表明亚铁绿铝石可能产生除最重的 GOE 前 Fe 同位素组成以外的所有成分,而混合价绿铝石可以产生整个记录。我们的结果表明,单独的重 Fe 同位素富集并不能诊断原生 IF 矿物学,而水铁矿和部分氧化或什至纯亚铁绿铝石都是可行的原生 IF 矿物学。
更新日期:2023-03-15
中文翻译:

太古代-早古元古代铁地层中初级沉淀物性质的同位素限制,来自绿铝石和 2L- 和 6L- 水铁矿状态的铁声子密度的测定
铁地层 (IFs) 是在大氧化事件 (GOE) 之前广泛沉积的化学沉积岩,大约 2.4–2.2 Ga。通常认为 IFs 在表面氧化后沉淀为水合 Fe 3+ 氧化物 (HFO),例如水铁矿富含Fe 2+的缺氧深水。该模型通常涉及生物氧化并支持海洋营养物浓度的重建。然而,纳米岩相学表明,Fe 2+硅酸盐绿岩是保存完好的 IFs 中常见的原生矿物,激发了缺氧硅酸亚铁沉淀的替代沉积模型。然而,尚不清楚 Fe 2+- 富含硅酸盐可以在 IF 中产生 Fe 同位素变化,这可以通过 Fe 2+氧化得到很好的解释。为了解决这个问题,我们通过确定这些矿物的铁声子态密度来限制绿铝石和水铁矿的平衡Fe 同位素 ( 56 Fe/ 54 Fe)分馏。我们使用从头算密度泛函理论 (DFT + U ) 计算和核共振非弹性 X 射线散射光谱表明,在平衡状态下,亚铁绿铁矿在同位素方面应比水铁矿轻 ~1–1.2‰,并且分馏应随 Fe 的增加呈线性变化3+绿岩中的含量。通过将水铁矿-绿铝石矿物对分馏锚定到已发表的 HFO 和 Fe 2+水溶液之间的实验性 Fe 同位素分馏,我们表明亚铁绿铝石可能产生除最重的 GOE 前 Fe 同位素组成以外的所有成分,而混合价绿铝石可以产生整个记录。我们的结果表明,单独的重 Fe 同位素富集并不能诊断原生 IF 矿物学,而水铁矿和部分氧化或什至纯亚铁绿铝石都是可行的原生 IF 矿物学。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号