当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pepstatin-Based Probes for Photoaffinity Labeling of Aspartic Proteases and Application to Target Identification
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2023-03-15 , DOI: 10.1021/acschembio.2c00946
Suyuan Chen 1 , Chunguang Liang 2, 3 , Hongli Li 4 , Weimeng Yu 2 , Michaela Prothiwa 5 , Dominik Kopczynski 6 , Stefan Loroch 1, 7, 8 , Marc Fransen 4 , Steven H L Verhelst 1, 5
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2023-03-15 , DOI: 10.1021/acschembio.2c00946
Suyuan Chen 1 , Chunguang Liang 2, 3 , Hongli Li 4 , Weimeng Yu 2 , Michaela Prothiwa 5 , Dominik Kopczynski 6 , Stefan Loroch 1, 7, 8 , Marc Fransen 4 , Steven H L Verhelst 1, 5
Affiliation
|
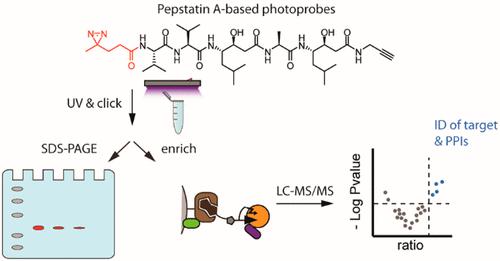
Aspartic proteases are a small class of proteases implicated in a wide variety of human diseases. Covalent chemical probes for photoaffinity labeling (PAL) of these proteases are underdeveloped. We here report a full on-resin synthesis of clickable PAL probes based on the natural product inhibitor pepstatin incorporating a minimal diazirine reactive group. The position of this group in the inhibitor determines the labeling efficiency. The most effective probes sensitively detect cathepsin D, a biomarker for breast cancer, in cell lysates. Moreover, through chemical proteomics experiments and deep learning algorithms, we identified sequestosome-1, an important player in autophagy, as a direct interaction partner and substrate of cathepsin D.
中文翻译:

用于天冬氨酸蛋白酶光亲和标记的基于胃酶抑素的探针及其在目标识别中的应用
天冬氨酸蛋白酶是一小类与多种人类疾病有关的蛋白酶。用于这些蛋白酶的光亲和标记 (PAL) 的共价化学探针尚未开发。我们在这里报告了基于天然产物抑制剂胃酶抑素的可点击 PAL 探针的完整树脂合成,并结合了最小的二氮杂环反应基团。该基团在抑制剂中的位置决定了标记效率。最有效的探针可灵敏地检测细胞裂解物中的组织蛋白酶 D,这是乳腺癌的生物标志物。此外,通过化学蛋白质组学实验和深度学习算法,我们确定了 sequestosome-1(自噬的重要参与者)作为组织蛋白酶 D 的直接相互作用伙伴和底物。
更新日期:2023-03-15
中文翻译:

用于天冬氨酸蛋白酶光亲和标记的基于胃酶抑素的探针及其在目标识别中的应用
天冬氨酸蛋白酶是一小类与多种人类疾病有关的蛋白酶。用于这些蛋白酶的光亲和标记 (PAL) 的共价化学探针尚未开发。我们在这里报告了基于天然产物抑制剂胃酶抑素的可点击 PAL 探针的完整树脂合成,并结合了最小的二氮杂环反应基团。该基团在抑制剂中的位置决定了标记效率。最有效的探针可灵敏地检测细胞裂解物中的组织蛋白酶 D,这是乳腺癌的生物标志物。此外,通过化学蛋白质组学实验和深度学习算法,我们确定了 sequestosome-1(自噬的重要参与者)作为组织蛋白酶 D 的直接相互作用伙伴和底物。































 京公网安备 11010802027423号
京公网安备 11010802027423号