当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revealing the Role of CO during CO2 Hydrogenation on Cu Surfaces with In Situ Soft X-Ray Spectroscopy
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-03-14 , DOI: 10.1021/jacs.2c12728 Jack E N Swallow 1 , Elizabeth S Jones 1 , Ashley R Head 2 , Joshua S Gibson 1 , Roey Ben David 3 , Michael W Fraser 1 , Matthijs A van Spronsen 4 , Shaojun Xu 5 , Georg Held 4 , Baran Eren 3 , Robert S Weatherup 1, 4
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-03-14 , DOI: 10.1021/jacs.2c12728 Jack E N Swallow 1 , Elizabeth S Jones 1 , Ashley R Head 2 , Joshua S Gibson 1 , Roey Ben David 3 , Michael W Fraser 1 , Matthijs A van Spronsen 4 , Shaojun Xu 5 , Georg Held 4 , Baran Eren 3 , Robert S Weatherup 1, 4
Affiliation
|
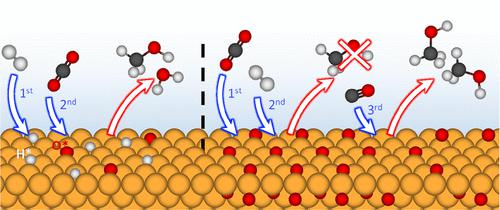
The reactions of H2, CO2, and CO gas mixtures on the surface of Cu at 200 °C, relevant for industrial methanol synthesis, are investigated using a combination of ambient pressure X-ray photoelectron spectroscopy (AP-XPS) and atmospheric-pressure near edge X-ray absorption fine structure (AtmP-NEXAFS) spectroscopy bridging pressures from 0.1 mbar to 1 bar. We find that the order of gas dosing can critically affect the catalyst chemical state, with the Cu catalyst maintained in a metallic state when H2 is introduced prior to the addition of CO2. Only on increasing the CO2 partial pressure is CuO formation observed that coexists with metallic Cu. When only CO2 is present, the surface oxidizes to Cu2O and CuO, and the subsequent addition of H2 partially reduces the surface to Cu2O without recovering metallic Cu, consistent with a high kinetic barrier to H2 dissociation on Cu2O. The addition of CO to the gas mixture is found to play a key role in removing adsorbed oxygen that otherwise passivates the Cu surface, making metallic Cu surface sites available for CO2 activation and subsequent conversion to CH3OH. These findings are corroborated by mass spectrometry measurements, which show increased H2O formation when H2 is dosed before rather than after CO2. The importance of maintaining metallic Cu sites during the methanol synthesis reaction is thereby highlighted, with the inclusion of CO in the gas feed helping to achieve this even in the absence of ZnO as the catalyst support.
中文翻译:

用原位软 X 射线光谱揭示铜表面 CO2 加氢过程中 CO 的作用
结合环境压力 X 射线光电子能谱 (AP-XPS) 和大气-研究了与工业甲醇合成相关的 200 °C 时 H 2、CO 2和 CO 气体混合物在 Cu 表面的反应近边压力 X 射线吸收精细结构 (AtmP-NEXAFS) 光谱桥接压力从 0.1 毫巴到 1 巴。我们发现气体计量的顺序可以严重影响催化剂的化学状态,当在添加 CO 2之前引入H 2时,Cu 催化剂保持在金属状态。只有在增加 CO 2分压时才观察到与金属 Cu 共存的 CuO 形成。当只有 CO 2存在时,表面氧化为 Cu 2 O 和 CuO,随后添加 H 2将表面部分还原为 Cu 2 O 而没有回收金属 Cu,这与Cu 2 O 上 H 2解离的高动力学势垒一致。添加发现气体混合物中的 CO 在去除吸附氧方面起着关键作用,否则会钝化 Cu 表面,使金属 Cu 表面位点可用于 CO 2活化并随后转化为 CH 3 OH。这些发现得到了质谱测量的证实,质谱测量显示当 H 2在 CO 之前而不是之后给药时,H 2 O 的形成增加2 . 因此强调了在甲醇合成反应过程中保持金属 Cu 位点的重要性,即使在没有 ZnO 作为催化剂载体的情况下,在气体进料中加入 CO 也有助于实现这一点。
更新日期:2023-03-14
中文翻译:

用原位软 X 射线光谱揭示铜表面 CO2 加氢过程中 CO 的作用
结合环境压力 X 射线光电子能谱 (AP-XPS) 和大气-研究了与工业甲醇合成相关的 200 °C 时 H 2、CO 2和 CO 气体混合物在 Cu 表面的反应近边压力 X 射线吸收精细结构 (AtmP-NEXAFS) 光谱桥接压力从 0.1 毫巴到 1 巴。我们发现气体计量的顺序可以严重影响催化剂的化学状态,当在添加 CO 2之前引入H 2时,Cu 催化剂保持在金属状态。只有在增加 CO 2分压时才观察到与金属 Cu 共存的 CuO 形成。当只有 CO 2存在时,表面氧化为 Cu 2 O 和 CuO,随后添加 H 2将表面部分还原为 Cu 2 O 而没有回收金属 Cu,这与Cu 2 O 上 H 2解离的高动力学势垒一致。添加发现气体混合物中的 CO 在去除吸附氧方面起着关键作用,否则会钝化 Cu 表面,使金属 Cu 表面位点可用于 CO 2活化并随后转化为 CH 3 OH。这些发现得到了质谱测量的证实,质谱测量显示当 H 2在 CO 之前而不是之后给药时,H 2 O 的形成增加2 . 因此强调了在甲醇合成反应过程中保持金属 Cu 位点的重要性,即使在没有 ZnO 作为催化剂载体的情况下,在气体进料中加入 CO 也有助于实现这一点。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号