当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dopant- and Surfactant-Tuned Electrode–Electrolyte Interface Enabling Efficient Alkynol Semi-Hydrogenation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-03-13 , DOI: 10.1021/jacs.3c00565 Yuan Zhao 1, 2 , Jipeng Xu 3 , Kai Huang 3 , Wangxin Ge 2 , Zhen Liu 1 , Cheng Lian 3 , Honglai Liu 3 , Hongliang Jiang 1 , Chunzhong Li 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-03-13 , DOI: 10.1021/jacs.3c00565 Yuan Zhao 1, 2 , Jipeng Xu 3 , Kai Huang 3 , Wangxin Ge 2 , Zhen Liu 1 , Cheng Lian 3 , Honglai Liu 3 , Hongliang Jiang 1 , Chunzhong Li 1, 2
Affiliation
|
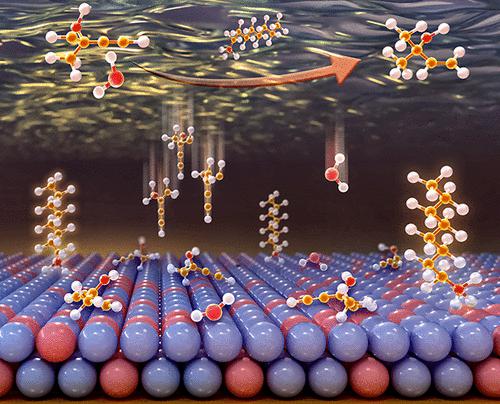
Electrochemical alkynol semi-hydrogenation has emerged as a sustainable and environmentally benign route for the production of high-value alkenols, featuring water as the hydrogen source instead of H2. It is highly challenging to design the electrode–electrolyte interface with efficient electrocatalysts and their matched electrolytes to break the selectivity-activity stereotype. Here, boron-doped Pd catalysts (PdB) and surfactant-modified interface are proposed to enable the simultaneous increase in alkenol selectivity and alkynol conversion. Typically, compared to pure Pd and commercial Pd/C catalysts, the PdB catalyst achieves both higher turnover frequency (139.8 h–1) and specific selectivity (above 90%) for the semi-hydrogenation of 2-methyl-3-butyn-2-ol (MBY). Quaternary ammonium cationic surfactants that are employed as electrolyte additives are assembled at the electrified interface in response to applied bias potential, establishing an interfacial microenvironment that can facilitate alkynol transfer and hinder water transfer suitably. Eventually the hydrogen evolution reaction is inhibited and alkynol semi-hydrogenation is promoted, without inducing the decrease of alkenol selectivity. This work offers a distinct perspective on creating a suitable electrode–electrolyte interface for electrosynthesis.
中文翻译:

掺杂剂和表面活性剂调节的电极-电解质界面可实现高效的炔醇半氢化
电化学炔醇半氢化已成为一种可持续且环境友好的高价值烯醇生产途径,其特征是水作为氢源而不是 H 2。设计具有高效电催化剂及其匹配电解质的电极-电解质界面以打破选择性-活性刻板印象是极具挑战性的。在这里,提出了掺硼 Pd 催化剂 (PdB) 和表面活性剂改性界面,以同时提高烯醇选择性和炔醇转化率。通常,与纯 Pd 和商用 Pd/C 催化剂相比,PdB 催化剂实现了更高的周转频率(139.8 h –1) 和 2-methyl-3-butyn-2-ol (MBY) 半氢化的特异性选择性(高于 90%)。用作电解质添加剂的季铵阳离子表面活性剂在带电界面上组装,以响应施加的偏置电位,从而建立可以促进炔醇转移并适当阻碍水转移的界面微环境。最终抑制了析氢反应,促进了炔醇的半加氢反应,而没有引起烯醇选择性的降低。这项工作为创建适合电合成的电极-电解质界面提供了独特的视角。
更新日期:2023-03-13
中文翻译:

掺杂剂和表面活性剂调节的电极-电解质界面可实现高效的炔醇半氢化
电化学炔醇半氢化已成为一种可持续且环境友好的高价值烯醇生产途径,其特征是水作为氢源而不是 H 2。设计具有高效电催化剂及其匹配电解质的电极-电解质界面以打破选择性-活性刻板印象是极具挑战性的。在这里,提出了掺硼 Pd 催化剂 (PdB) 和表面活性剂改性界面,以同时提高烯醇选择性和炔醇转化率。通常,与纯 Pd 和商用 Pd/C 催化剂相比,PdB 催化剂实现了更高的周转频率(139.8 h –1) 和 2-methyl-3-butyn-2-ol (MBY) 半氢化的特异性选择性(高于 90%)。用作电解质添加剂的季铵阳离子表面活性剂在带电界面上组装,以响应施加的偏置电位,从而建立可以促进炔醇转移并适当阻碍水转移的界面微环境。最终抑制了析氢反应,促进了炔醇的半加氢反应,而没有引起烯醇选择性的降低。这项工作为创建适合电合成的电极-电解质界面提供了独特的视角。

































 京公网安备 11010802027423号
京公网安备 11010802027423号