Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2023-03-11 , DOI: 10.1016/j.apcatb.2023.122617 Hongjie Yu , Wenxin Wang , Qiqi Mao , Kai Deng , Ziqiang Wang , You Xu , Xiaonian Li , Hongjing Wang , Liang Wang
|
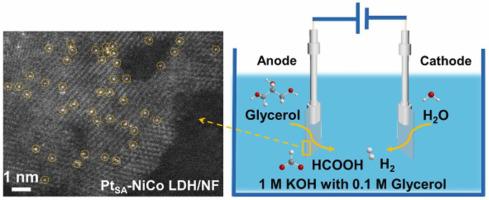
By combining hydrogen evolution reaction (HER) with glycerol oxidation reaction (GOR), high-value chemicals can be obtained at the anode while saving energy to produce hydrogen. Herein, we synthesized PtSA-NiCo LDHs/NF by anchoring oxygen vacancies in NiCo LDHs with Pt to form Pt-O5 coordination. Density functional theory (DFT) revealed that PtSA reduced the d-band center and optimized ΔGH*. During HER, LDHs act as active sites to catalyze H-OH cleavage and promote the dissociation of H2O, while Pt single atoms act as the binding sites for H intermediates, and Pt-O bonds accelerate proton-electron coupling and promote the release of H2 molecules. In HER//GOR, a cell voltage of only 1.37 V is required to provide a current density of 100 mA cm−2 and to produce formate at the anode. This work enables energy-saving hydrogen production and high-value chemicals at the anode, a novel green electrocatalytic synthesis strategy with synergistic coupling and dual promotion.
中文翻译:

富含氧空位的 NiCo 层状双氢氧化物捕获 Pt 单原子,用于耦合析氢与甘油选择性氧化成甲酸盐
通过将析氢反应(HER)与甘油氧化反应(GOR)相结合,可以在阳极获得高价值的化学物质,同时节约能源制氢。在此,我们通过用 Pt 锚定 NiCo LDHs 中的氧空位形成 Pt-O 5配位来合成 Pt SA -NiCo LDHs/NF 。密度泛函理论 (DFT) 表明 Pt SA降低了d带中心并优化了 ΔG H*。在HER过程中,LDHs作为活性位点催化H-OH裂解,促进H 2 O的解离,而Pt单原子作为H中间体的结合位点,Pt-O键加速质子-电子耦合并促进释放氢2分子。在 HER//GOR 中,仅需要 1.37 V 的电池电压即可提供 100 mA cm -2的电流密度并在阳极产生甲酸盐。这项工作能够在阳极实现节能制氢和高价值化学品,这是一种具有协同耦合和双重促进作用的新型绿色电催化合成策略。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号