当前位置:
X-MOL 学术
›
Green Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Base-controlled annulation of tryptamine-derived isocyanides with nitrile imines for access to polycyclic spiroindoline derivatives
Green Synthesis and Catalysis ( IF 8.2 ) Pub Date : 2022-06-11 , DOI: 10.1016/j.gresc.2022.06.001
Xiaofeng Wang , Luorong Yuan , Xiaoping Xu , Shunjun Ji
中文翻译:

色胺衍生的异氰化物与腈亚胺的碱基控制环化以获得多环螺二氢吲哚衍生物
更新日期:2022-06-11
Green Synthesis and Catalysis ( IF 8.2 ) Pub Date : 2022-06-11 , DOI: 10.1016/j.gresc.2022.06.001
Xiaofeng Wang , Luorong Yuan , Xiaoping Xu , Shunjun Ji
|
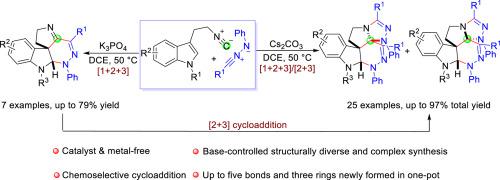
An annulation reaction of tryptamine-derived isocyanides with hydrazonyl chlorides in the presence of bases was developed. Controlled by different bases, [1+ 2+3] annulation and [1+ 2+3]/[2 + 3] annulation cascade were realized. In the latter reaction, five new chemical bonds as well as three new heterocycles were formed in one step. It showed extremely high efficiency, relatively broad substrate scope, milder reaction conditions, good tolerance of functional groups and good chemoselectivity.
中文翻译:

色胺衍生的异氰化物与腈亚胺的碱基控制环化以获得多环螺二氢吲哚衍生物
开发了色胺衍生的异氰化物与肼基氯在碱存在下的环化反应。在不同碱基的控制下,实现了[1+ 2+3]环化和[1+ 2+3]/[2 + 3]环化级联。在后一反应中,一步形成了五个新的化学键和三个新的杂环。它表现出极高的效率、相对广泛的底物范围、较温和的反应条件、良好的官能团耐受性和良好的化学选择性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号