Science of the Total Environment ( IF 8.2 ) Pub Date : 2023-03-11 , DOI: 10.1016/j.scitotenv.2023.162734 Li Wang 1 , Lantian Zhou 1 , Longyu Liu 1 , Yu Yang 1 , Qiang Zhao 1
|
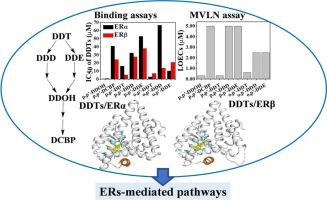
DDT and its transformation products (DDTs) are frequently detected in environmental and biological media. Research suggests that DDT and its primary metabolites (DDD and DDE) could induce estrogenic effects by disturbing estrogen receptor (ER) pathways. However, the estrogenic effects of DDT high-order transformation products, and the exact mechanisms underlying the differences of responses in DDT and its metabolites (or transformation products) still remain unknown. Here, besides DDT, DDD and DDE, we selected two DDT high-order transformation products, 2,2-bis(4-chlorophenyl) ethanol (p,p′-DDOH) and 4,4′-dichlorobenzophenone (p,p′-DCBP). We aim to explore and reveal the relation between DDTs activity and their estrogenic effects by receptor binding, transcriptional activity, and ER-mediated pathways. Fluorescence assays showed that the tested 8 DDTs bound to the two isoforms (ERα and ERβ) of ER directly. Among them, p,p′-DDOH exhibited the highest binding affinity, with IC50 values of 0.43 μM and 0.97 μM to ERα and ERβ, respectively. Eight DDTs showed different agonistic activity toward ER pathways, with p,p′-DDOH exhibiting the strongest potency. In silico studies revealed that the eight DDTs bound to either ERα or ERβ in a similar manner to 17β-estradiol, in which specific polar and non-polar interactions and water-mediated hydrogen bonds were involved. Furthermore, we found that 8 DDTs (0.0008–5 μM) showed distinct pro-proliferative effects on MCF-7 cells in an ER-dependent manner. Overall, our results revealed not only for the first time the estrogenic effects of two DDT high-order transformation products by acting on ER-mediated pathways, but also the molecular basis for differential activity of 8 DDTs.
中文翻译:

2,2-双(4-氯苯基)乙醇、4,4'-二氯二苯甲酮和 DDT 类似物雌激素作用的体外和计算机比较研究
DDT 及其转化产物 (DDT) 经常在环境和生物介质中检测到。研究表明,DDT 及其主要代谢物(DDD 和 DDE)可通过干扰雌激素受体 (ER) 途径诱导雌激素作用。然而,DDT 高阶转化产物的雌激素效应,以及 DDT 及其代谢产物(或转化产物)反应差异的确切机制仍然未知。在这里,除了DDT、DDD和DDE之外,我们还选择了两种DDT高阶转化产物,2,2-双(4-氯苯基)乙醇( p,p' -DDOH)和4,4'-二氯二苯甲酮( p,p'-DCBP)。我们旨在通过受体结合、转录活性和 ER 介导的途径探索和揭示 DDT 活性与其雌激素作用之间的关系。荧光测定表明,所测试的 8 种 DDT 直接与 ER 的两种亚型(ERα 和 ERβ)结合。其中,p,p' -DDOH 表现出最高的结合亲和力,对 ERα 和 ERβ 的IC 50值分别为 0.43 μM 和 0.97 μM。八种 DDT 对 ER 通路表现出不同的激动活性,p,p′-DDOH 表现出最强的效力。计算机研究表明,八种 DDT 以与 17β-雌二醇相似的方式与 ERα 或 ERβ 结合,其中涉及特定的极性和非极性相互作用以及水介导的氢键。此外,我们发现 8 种 DDT (0.0008–5 μM) 以 ER 依赖性方式对 MCF-7 细胞显示出明显的促增殖作用。总体而言,我们的结果不仅首次揭示了两种 DDT 高阶转化产物通过作用于 ER 介导的途径而产生的雌激素效应,而且还揭示了 8 种 DDT 差异活性的分子基础。






























 京公网安备 11010802027423号
京公网安备 11010802027423号