Molecular Catalysis ( IF 3.9 ) Pub Date : 2023-03-07 , DOI: 10.1016/j.mcat.2023.113055 Qiang Chen , Hu Xiao , Zhi-Pu Li , Xiao-Qiong Pei , Wen Yang , Yan Liu , Zhong-Liu Wu
|
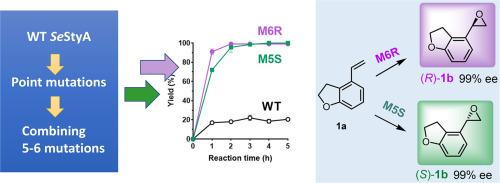
The asymmetric epoxidation of unfunctionalized terminal olefins remains a challenge. Styrene monooxygenase SeStyA from Streptomyces exfoliatus could catalyze the enantioselective epoxidation of terminal olefins. To improve the robustness of SeStyA, a consensus-based approach was applied, which led to the successful identification of several beneficial mutations with enhanced thermostability or enantioselectivity in the epoxidation of 4-vinyl-2,3-dihydrobenzofuran (1a), a Tasimelteon intermediate. After sequential combinatory mutagenesis, mutants M6R and M5S with complementary enantioselectivity were selected to catalyze respectively the (R)- and (S)-epoxidation of 1a, generating both enantiomers with 99% ee. The inactivation half-life of M6R and M5S at 40 °C was increased to 25.4 and 5.9-fold of the wild-type, respectively. Both can catalyze the complete epoxidation of 20 mM substrate within 2 to 3 h, a significant improvement from the 20% conversion of the wild-type. The results were interpreted in the context of the model structure of SeStyA.
中文翻译:

使用具有增强稳定性和对映选择性的 SeStyA 突变体对 4-乙烯基-2,3-二氢苯并呋喃进行立体互补环氧化
未官能化末端烯烃的不对称环氧化仍然是一个挑战。来自Streptomyces exfoliatus 的苯乙烯单加氧酶Se StyA可以催化末端烯烃的对映选择性环氧化。为了提高Se StyA的稳健性,应用了一种基于共识的方法,成功鉴定了几种有益突变,这些突变在 4-乙烯基-2,3-二氢苯并呋喃 ( 1a ) 的环氧化反应中具有增强的热稳定性或对映选择性,他司美琼中间的。经过顺序组合诱变,选择具有互补对映选择性的突变体 M6R 和 M5S 分别催化1a的( R )- 和 ( S )- 环氧化反应, 产生 99% ee 的两种对映体。M6R 和 M5S 在 40 °C 的灭活半衰期分别增加到野生型的 25.4 倍和 5.9 倍。两者都可以在 2 至 3 小时内催化 20 mM 底物的完全环氧化,与野生型的 20% 转化率相比有了显着提高。结果是在Se StyA 模型结构的背景下解释的。






























 京公网安备 11010802027423号
京公网安备 11010802027423号