Tetrahedron ( IF 2.1 ) Pub Date : 2023-03-09 , DOI: 10.1016/j.tet.2023.133363 Marek Kõllo , Kristi Rõuk , Ivar Järving , Tõnis Pehk , Margus Lopp
|
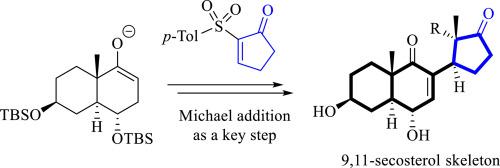
The application of Michael addition to the construction of the carbon skeleton of 9,11-secosterols has been investigated using the following Michael acceptor - sulfone 2-[(4-methylphenyl)sulfonyl]cyclopent-2-en-1-one, where the addition product was isolated in good yield and as a mixture of two diastereomers. Also, the diastereomers were separable by crystallization, and, based on NMR spectroscopic data, the relative configuration of the formed stereocentres of the isolated diastereomer matched the 9,11-secosterol found in nature. This study can be exploited to create a total synthesis scheme for 9,11-secosterols.
中文翻译:

迈向 9,11-secosterol 的全合成:将 A、B 和 D 环与迈克尔加成连接到砜激活的环戊烯酮
已使用以下迈克尔受体-砜 2-[(4-甲基苯基)磺酰基]环戊二烯-2-en-1-one 研究了迈克尔加成在构建 9,11-secosterols 碳骨架中的应用,其中加成产物以良好的收率分离,并且是两种非对映异构体的混合物。此外,非对映异构体可通过结晶分离,并且根据 NMR 光谱数据,分离的非对映异构体形成的立体中心的相对构型与自然界中发现的 9,11-仲甾醇相匹配。该研究可用于创建 9,11-二甾醇的全合成方案。































 京公网安备 11010802027423号
京公网安备 11010802027423号