Nano Today ( IF 13.2 ) Pub Date : 2023-03-11 , DOI: 10.1016/j.nantod.2023.101821 Chuangui Sheng , Fangzhi Yu , Youming Feng , Ming Gao , Wei Li , Lele Li , Jian Zhao
|
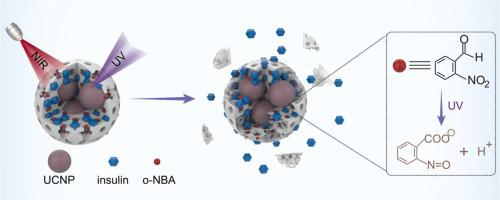
Metal−organic frameworks (MOFs) have emerged as a promising platform for protein delivery due to their high loading capacity and tunable functionality. However, spatiotemporally-controlled release of loaded proteins from MOFs-based nanocarriers remains an outstanding challenge. Herein, we report a MOFs-based protein delivery system that is capable of precisely controlling protein release with deep tissue penetrable near-infrared (NIR) light. The responsive system was constructed by encapsulating a protein of interest and upconversion nanoparticles (UCNPs) within zeolitic imidazolate framework-8 (ZIF-8) MOFs and further trapping a photoacid generator (PAG) in the pores of ZIF-8. Upon NIR light irradiation, UCNPs emit ultraviolet light that activates PAG to produce protons, which enables local pH acidification and thus degradation of ZIF-8 for spatiotemporally-controlled protein release. By employing insulin as a model protein, we demonstrate that the system allows for on-demand control over its therapeutic efficacy against diabetes with NIR light. This work illustrates a general strategy to regulate MOFs-based nanocarriers for controlled drug delivery.
中文翻译:

近红外光触发金属有机框架的降解以实现时空控制的蛋白质释放
金属有机框架 (MOF) 由于其高负载能力和可调功能,已成为一种很有前途的蛋白质递送平台。然而,时空控制地从基于 MOF 的纳米载体中释放负载的蛋白质仍然是一个突出的挑战。在此,我们报告了一种基于 MOFs 的蛋白质递送系统,该系统能够通过深层组织可穿透的近红外 (NIR) 光精确控制蛋白质释放。通过将目标蛋白和上转换纳米粒子 (UCNP) 封装在沸石咪唑酯骨架 8 (ZIF-8) MOF 中,并进一步将光酸生成剂 (PAG) 捕获在 ZIF-8 的孔隙中,构建了响应系统。在 NIR 光照射下,UCNP 发出紫外光,激活 PAG 产生质子,这使得局部 pH 酸化,从而使 ZIF-8 降解以实现时空控制的蛋白质释放。通过使用胰岛素作为模型蛋白质,我们证明该系统允许按需控制其使用 NIR 光对糖尿病的治疗效果。这项工作说明了调节基于 MOFs 的纳米载体以控制药物输送的一般策略。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号