Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2023-03-10 , DOI: 10.1016/j.bmcl.2023.129235 Fei Liu 1 , Bin Wang 2 , Yanlong Liu 2 , Wei Shi 2 , Zhongyuan Hu 2 , Xiayun Chang 2 , Xujing Tang 2 , Ying Zhang 2 , Hongjiang Xu 2 , Ying He 3
|
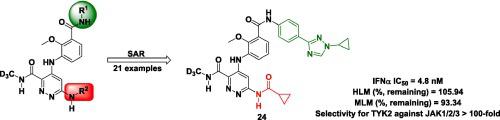
As a mediator of pro-inflammatory cytokines, TYK2 is an attractive target to treat autoimmunity diseases. Herein, we reported the design, synthesis, and structure–activity relationships (SARs) of N-(methyl-d3) pyridazine-3-carboxamide derivatives as TYK2 inhibitors. Among them, compound 24 exhibited acceptable inhibition activity against STAT3 phosphorylation. Furthermore, 24 showed satisfactory selectivities toward other members of JAK family and performed a good stability profile in liver microsomal assay. Pharmacokinetics (PK) study indicated that compound 24 has reasonable PK exposures. In anti-CD40-induced colitis models, compound 24 was orally highly effective with no significant hERG and CYP isozymes inhibition. These results indicated that compound 24 was worthy of further investigation for the development of anti-autoimmunity diseases agents.
中文翻译:

新型 N-(甲基-d3) 哒嗪-3-甲酰胺衍生物作为 TYK2 抑制剂的设计、合成和生物学评价
作为促炎细胞因子的介质,TYK2 是治疗自身免疫性疾病的一个有吸引力的靶点。在此,我们报道了N -(methyl- d 3 ) 哒嗪-3-甲酰胺衍生物作为 TYK2 抑制剂的设计、合成和构效关系 (SAR) 。其中,化合物24对STAT3磷酸化表现出可接受的抑制活性。此外,24对 JAK 家族的其他成员表现出令人满意的选择性,并且在肝微粒体测定中表现出良好的稳定性。药代动力学 (PK) 研究表明化合物24具有合理的 PK 暴露。在抗 CD40 诱导的结肠炎模型中,化合物24口服非常有效,没有显着的 hERG 和 CYP 同工酶抑制作用。这些结果表明化合物24值得进一步研究用于开发抗自身免疫性疾病药物。































 京公网安备 11010802027423号
京公网安备 11010802027423号