当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Why 6-Iodouridine Cannot Be Used as a Radiosensitizer of DNA Damage? Computational and Experimental Studies
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2023-03-09 , DOI: 10.1021/acs.jpcb.3c00548 Karina Falkiewicz 1 , Witold Kozak 1 , Magdalena Zdrowowicz 1 , Paulina Spisz 1, 2 , Lidia Chomicz-Mańka 1 , Mieczyslaw Torchala 1 , Janusz Rak 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2023-03-09 , DOI: 10.1021/acs.jpcb.3c00548 Karina Falkiewicz 1 , Witold Kozak 1 , Magdalena Zdrowowicz 1 , Paulina Spisz 1, 2 , Lidia Chomicz-Mańka 1 , Mieczyslaw Torchala 1 , Janusz Rak 1
Affiliation
|
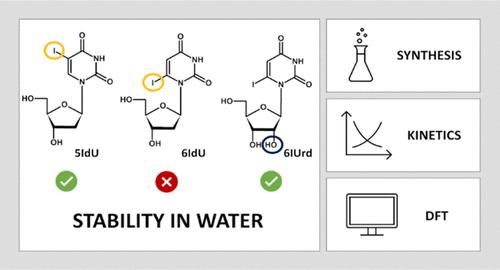
Previous density functional theory (DFT) studies on 6-brominated pyrimidine nucleosides suggest that 6-iodo-2′-deoxyuridine (6IdU) should act as a better radiosensitizer than its 5-iodosubstituted 2′-deoxyuridine analogue. In this work, we show that 6IdU is unstable in an aqueous solution. Indeed, a complete disappearance of the 6IdU signal was observed during its isolation by reversed-phase high-performance liquid chromatography (RP-HPLC). As indicated by the thermodynamic characteristics for the SN1-type hydrolysis of 6IdU obtained at the CAM-B3LYP/DGDZVP++ level and the polarizable continuum model (PCM) of water, 6-iodouracil (6IU) was already released quantitatively at ambient temperatures. The simulation of the hydrolysis kinetics demonstrated that a thermodynamic equilibrium was reached within seconds for the title compound. To assess the reliability of the calculations carried out, we synthesized 6-iodouridine (6IUrd), which was, unlike 6IdU, sufficiently stable in an aqueous solution at room temperature. The activation barrier for the N-glycosidic bond dissociation in 6IUrd was estimated experimentally using an Arrhenius plot. The stabilities in water calculated for 6IdU, 6IUrd, and 5-iodo-2′-deoxyuridine (5IdU) could be explained by the electronic and steric effects of the 2′-hydroxy group present in the ribose moiety. Our studies highlight the issue of the hydrolytic stability of potentially radiosensitizing nucleotides which, besides having favorable dissociative electron attachment (DEA) characteristics, must be stable in water to have any practical application.
中文翻译:

为什么6-碘尿苷不能作为DNA损伤的放射增敏剂?计算和实验研究
先前对 6-溴化嘧啶核苷的密度泛函理论 (DFT) 研究表明,6-碘-2'-脱氧尿苷 (6IdU) 应该比其 5-碘代 2'-脱氧尿苷类似物作为更好的放射增敏剂。在这项工作中,我们表明 6IdU 在水溶液中不稳定。实际上,在通过反相高效液相色谱 (RP-HPLC) 分离过程中观察到 6IdU 信号完全消失。正如 S N的热力学特性所示在 CAM-B3LYP/DGDZVP++ 水平和水的可极化连续模型 (PCM) 中获得的 6IdU 的 1 型水解,6-碘尿嘧啶 (6IU) 已经在环境温度下定量释放。水解动力学模拟表明,标题化合物在数秒内达到热力学平衡。为了评估所进行计算的可靠性,我们合成了 6-碘尿苷 (6IUrd),与 6IdU 不同,它在室温下在水溶液中足够稳定。N的激活势垒6IUrd 中的糖苷键解离是使用 Arrhenius 图通过实验估计的。6IdU、6IUrd 和 5-iodo-2'-脱氧尿苷 (5IdU) 在水中的稳定性可以通过核糖部分中存在的 2'-羟基的电子和空间效应来解释。我们的研究强调了潜在放射增敏核苷酸的水解稳定性问题,这些核苷酸除了具有有利的解离电子附着 (DEA) 特性外,还必须在水中稳定才能具有任何实际应用。
更新日期:2023-03-09
中文翻译:

为什么6-碘尿苷不能作为DNA损伤的放射增敏剂?计算和实验研究
先前对 6-溴化嘧啶核苷的密度泛函理论 (DFT) 研究表明,6-碘-2'-脱氧尿苷 (6IdU) 应该比其 5-碘代 2'-脱氧尿苷类似物作为更好的放射增敏剂。在这项工作中,我们表明 6IdU 在水溶液中不稳定。实际上,在通过反相高效液相色谱 (RP-HPLC) 分离过程中观察到 6IdU 信号完全消失。正如 S N的热力学特性所示在 CAM-B3LYP/DGDZVP++ 水平和水的可极化连续模型 (PCM) 中获得的 6IdU 的 1 型水解,6-碘尿嘧啶 (6IU) 已经在环境温度下定量释放。水解动力学模拟表明,标题化合物在数秒内达到热力学平衡。为了评估所进行计算的可靠性,我们合成了 6-碘尿苷 (6IUrd),与 6IdU 不同,它在室温下在水溶液中足够稳定。N的激活势垒6IUrd 中的糖苷键解离是使用 Arrhenius 图通过实验估计的。6IdU、6IUrd 和 5-iodo-2'-脱氧尿苷 (5IdU) 在水中的稳定性可以通过核糖部分中存在的 2'-羟基的电子和空间效应来解释。我们的研究强调了潜在放射增敏核苷酸的水解稳定性问题,这些核苷酸除了具有有利的解离电子附着 (DEA) 特性外,还必须在水中稳定才能具有任何实际应用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号