当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electronic and Lattice Engineering of Ruthenium Oxide towards Highly Active and Stable Water Splitting
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2023-03-08 , DOI: 10.1002/aenm.202300177 Liqiang Hou 1 , Zijian Li 2 , Haeseong Jang 3 , Yu wang 1 , Xuemei Cui 1 , Xiumin Gu 1 , Min Gyu Kim 4 , Ligang Feng 5 , Shangguo Liu 1 , Xien Liu 1
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2023-03-08 , DOI: 10.1002/aenm.202300177 Liqiang Hou 1 , Zijian Li 2 , Haeseong Jang 3 , Yu wang 1 , Xuemei Cui 1 , Xiumin Gu 1 , Min Gyu Kim 4 , Ligang Feng 5 , Shangguo Liu 1 , Xien Liu 1
Affiliation
|
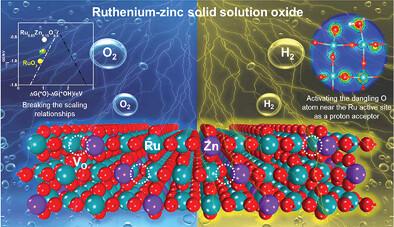
The development of efficiently active and stable bifunctional noble-metal-based electrocatalysts toward overall water splitting is urgent and challenging. In this work, a rutile-structured ruthenium-zinc solid solution oxide with oxygen vacancies (Ru0.85Zn0.15O2-δ) is developed by a simple molten salt method. With naturally abundant edges of ultrasmall nanoparticles clusters, Ru0.85Zn0.15O2-δ requires ultralow overpotentials, 190 mV for acidic oxygen evolution reaction (OER) and 14 mV for alkaline hydrogen evolution reaction (HER), to reach 10 mA cm−2. Moreover, it shows superior activity and durability for overall water splitting in different electrolytes. Experimental characterizations and density functional theory calculations indicate that the incorporation of Zn and oxygen vacancies can optimize the electronic structure of RuO2 by charge redistribution, which dramatically suppresses the generation of soluble Rux>4 and allows optimized adsorption energies of oxygen intermediates for OER. Meanwhile, the incorporation of Zn can distort local structure to activate the dangling O atoms on the distorted Ru0.85Zn0.15O2-δ as proton acceptors, which firmly bonds the H atom in H2O* to stabilize the H2O and considerably improves the HER activity.
中文翻译:

氧化钌的电子和晶格工程实现高活性和稳定的水分解
开发高效活性和稳定的双功能贵金属基电催化剂以实现全水分解是紧迫且具有挑战性的。在这项工作中,通过简单的熔盐法开发了具有氧空位的金红石结构的钌-锌固溶体氧化物 (Ru 0.85 Zn 0.15 O 2-δ )。Ru 0.85 Zn 0.15 O 2-δ具有天然丰富的超小纳米粒子簇边缘,需要超低过电位,酸性析氧反应 (OER) 为 190 mV,碱性析氢反应 (HER) 为 14 mV,以达到 10 mA cm −2. 此外,它在不同电解质中的整体水分解表现出优异的活性和耐久性。实验表征和密度泛函理论计算表明,Zn 和氧空位的结合可以通过电荷再分配优化 RuO 2的电子结构,从而显着抑制可溶性 Ru x >4的产生,并优化 OER 氧中间体的吸附能。同时,Zn的掺入可以扭曲局部结构,激活扭曲的Ru 0.85 Zn 0.15 O 2-δ上的悬空O原子作为质子受体,牢固地结合H 2 O*中的H原子,稳定H 2O 并大大提高了 HER 活性。
更新日期:2023-03-08
中文翻译:

氧化钌的电子和晶格工程实现高活性和稳定的水分解
开发高效活性和稳定的双功能贵金属基电催化剂以实现全水分解是紧迫且具有挑战性的。在这项工作中,通过简单的熔盐法开发了具有氧空位的金红石结构的钌-锌固溶体氧化物 (Ru 0.85 Zn 0.15 O 2-δ )。Ru 0.85 Zn 0.15 O 2-δ具有天然丰富的超小纳米粒子簇边缘,需要超低过电位,酸性析氧反应 (OER) 为 190 mV,碱性析氢反应 (HER) 为 14 mV,以达到 10 mA cm −2. 此外,它在不同电解质中的整体水分解表现出优异的活性和耐久性。实验表征和密度泛函理论计算表明,Zn 和氧空位的结合可以通过电荷再分配优化 RuO 2的电子结构,从而显着抑制可溶性 Ru x >4的产生,并优化 OER 氧中间体的吸附能。同时,Zn的掺入可以扭曲局部结构,激活扭曲的Ru 0.85 Zn 0.15 O 2-δ上的悬空O原子作为质子受体,牢固地结合H 2 O*中的H原子,稳定H 2O 并大大提高了 HER 活性。































 京公网安备 11010802027423号
京公网安备 11010802027423号