当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lithium-Directed Transformation of Amorphous Iridium (Oxy)hydroxides To Produce Active Water Oxidation Catalysts
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-03-09 , DOI: 10.1021/jacs.2c13567
Jonathan Ruiz Esquius 1, 2 , David J Morgan 1 , Gerardo Algara Siller 3 , Diego Gianolio 4 , Matteo Aramini 4 , Leopold Lahn 5, 6 , Olga Kasian 5, 6 , Simon A Kondrat 7 , Robert Schlögl 3, 8 , Graham J Hutchings 1 , Rosa Arrigo 9 , Simon J Freakley 10
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-03-09 , DOI: 10.1021/jacs.2c13567
Jonathan Ruiz Esquius 1, 2 , David J Morgan 1 , Gerardo Algara Siller 3 , Diego Gianolio 4 , Matteo Aramini 4 , Leopold Lahn 5, 6 , Olga Kasian 5, 6 , Simon A Kondrat 7 , Robert Schlögl 3, 8 , Graham J Hutchings 1 , Rosa Arrigo 9 , Simon J Freakley 10
Affiliation
|
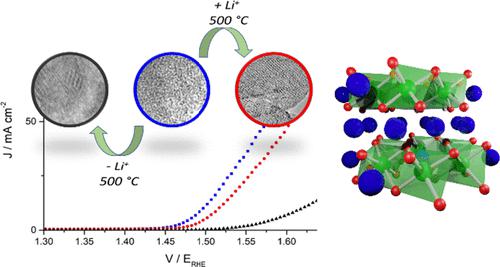
The oxygen evolution reaction (OER) is crucial to future energy systems based on water electrolysis. Iridium oxides are promising catalysts due to their resistance to corrosion under acidic and oxidizing conditions. Highly active iridium (oxy)hydroxides prepared using alkali metal bases transform into low activity rutile IrO2 at elevated temperatures (>350 °C) during catalyst/electrode preparation. Depending on the residual amount of alkali metals, we now show that this transformation can result in either rutile IrO2 or nano-crystalline Li-intercalated IrOx. While the transition to rutile results in poor activity, the Li-intercalated IrOx has comparative activity and improved stability when compared to the highly active amorphous material despite being treated at 500 °C. This highly active nanocrystalline form of lithium iridate could be more resistant to industrial procedures to produce PEM membranes and provide a route to stabilize the high populations of redox active sites of amorphous iridium (oxy)hydroxides.
中文翻译:

无定形铱(氧)氢氧化物的锂定向转化生产活性水氧化催化剂
析氧反应 (OER) 对于未来基于水电解的能源系统至关重要。氧化铱因其在酸性和氧化条件下的耐腐蚀性而成为很有前途的催化剂。在催化剂/电极制备过程中,使用碱金属碱制备的高活性铱(氧)氢氧化物在高温 (>350 °C) 下转化为低活性金红石 IrO 2 。根据碱金属的残留量,我们现在表明这种转变可以产生金红石IrO 2或纳米晶锂嵌入 IrO x。虽然过渡到金红石会导致活性差,但嵌入锂的 IrO x尽管在 500 °C 下处理,但与高活性无定形材料相比,具有相当的活性和改进的稳定性。这种高度活性的纳米晶体形式的铱酸锂可能更能抵抗生产 PEM 膜的工业程序,并提供稳定无定形氢氧化铱(氧)氧化物的大量氧化还原活性位点的途径。
更新日期:2023-03-09
中文翻译:

无定形铱(氧)氢氧化物的锂定向转化生产活性水氧化催化剂
析氧反应 (OER) 对于未来基于水电解的能源系统至关重要。氧化铱因其在酸性和氧化条件下的耐腐蚀性而成为很有前途的催化剂。在催化剂/电极制备过程中,使用碱金属碱制备的高活性铱(氧)氢氧化物在高温 (>350 °C) 下转化为低活性金红石 IrO 2 。根据碱金属的残留量,我们现在表明这种转变可以产生金红石IrO 2或纳米晶锂嵌入 IrO x。虽然过渡到金红石会导致活性差,但嵌入锂的 IrO x尽管在 500 °C 下处理,但与高活性无定形材料相比,具有相当的活性和改进的稳定性。这种高度活性的纳米晶体形式的铱酸锂可能更能抵抗生产 PEM 膜的工业程序,并提供稳定无定形氢氧化铱(氧)氧化物的大量氧化还原活性位点的途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号