Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2023-03-05 , DOI: 10.1016/j.molstruc.2023.135283 Rafik Saddik , Silvia A. Brandán , Salma Mortada , Cemile Baydere , Othmane Roby , Necmi Dege , Said Tighadouini , Mohamed Tahiri , My Abbes Faouzi , Khalid Karrouchi
|
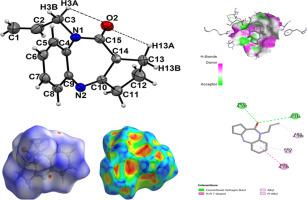
In this work, a novel benzodiazepine-2-one derivative (4) was synthesized by N-allylation of cyclopenta[e][1,5]benzodiazepine-2-one. The chemical structure of the target compound was confirmed by FT-IR, UV-Vis, 1H & 13C-NMR, GC-MS and single-crystal X-ray diffraction methods. Structural, topological, electronic and reactivity of new molecule were studied by using B3LYP/6-311++G** calculations in ethanol and in gas phase. Studies on charges, molecular electrostatic potentials, atoms in molecules (AIM), natural bond orbital (NBO), and frontier orbitals have evidenced that the seven membered diazepine ring play a very important role in the properties of (4) increasing its reactivity in ethanol. Good concordances are observed when the predicted IR, 1H-NMR, 13C-NMR and UV-Vis spectra are compared with the corresponding experimental ones. Complete vibrational assignments together with the scaled force constants are reported. The new compound was evaluated in vitro for their antihyperglycemic activity against α-amylase and α-glucosidase enzymes. Compound 4 was found to be more potent with IC50 value of 30.63 μM against α-glucosidase enzyme, compared to the reference drug “Acarbose” (IC50 = 113.60 μM) and exhibited good inhibitory activity against α-amylase enzyme with IC50 value of 133.8 μM as compared with the reference drug “Acarbose” (IC50 = 124.5 μM). Additionally, in silico molecular docking studies were carried out to confirm the experimental observations and investigate the efficacy of the title compound.
中文翻译:

9-allyl-2,3,9,10a-tetrahydrobenzo[b]cyclopenta[e][1,4]diazepin-10(1H)-one 的合成、晶体结构、Hirshfeld 表面分析、DFT 和降糖活性
在这项工作中,通过环戊二烯 [e][1,5] 苯二氮卓-2-酮的 N-烯丙基化合成了一种新型苯并二氮卓-2-酮衍生物 (4)。目标化合物的化学结构通过FT-IR、UV-Vis、 1 H & 13 C-NMR、GC-MS和单晶X-ray衍射等方法确证。通过在乙醇和气相中使用 B3LYP/6-311++G** 计算研究了新分子的结构、拓扑、电子和反应性。对电荷、分子静电势、分子中原子 (AIM)、自然键轨道 (NBO) 和前沿轨道的研究表明,七元二氮卓环在 (4) 提高其在乙醇中的反应性方面发挥着非常重要的作用. 当预测的 IR、1 H-NMR、13 C-NMR和UV-Vis光谱与相应的实验光谱进行了比较。报告了完整的振动分配以及缩放的力常数。在体外评估了新化合物对 α-淀粉酶和 α-葡萄糖苷酶的抗高血糖活性。与参考药物“阿卡波糖”(IC 50 = 113.60 μM)相比,发现化合物 4 对 α-葡萄糖苷酶的IC 50值为 30.63 μM更有效,并且对具有 IC 50值的 α-淀粉酶表现出良好的抑制活性与参考药物“阿卡波糖”(IC 50 = 124.5 μM)相比为 133.8 μM。此外,在计算机中进行分子对接研究以证实实验观察结果并研究标题化合物的功效。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号