当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rapid Redox Cycling of Fe(II)/Fe(III) in Microdroplets during Iron–Citric Acid Photochemistry
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-03-08 , DOI: 10.1021/acs.est.2c07897 Jinzhao Wang 1, 2 , Di Huang 1, 2 , Fengxia Chen 1, 2 , Jianhua Chen 1, 2 , Hongyu Jiang 1, 2 , Yifan Zhu 1, 2 , Chuncheng Chen 1, 2 , Jincai Zhao 1, 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-03-08 , DOI: 10.1021/acs.est.2c07897 Jinzhao Wang 1, 2 , Di Huang 1, 2 , Fengxia Chen 1, 2 , Jianhua Chen 1, 2 , Hongyu Jiang 1, 2 , Yifan Zhu 1, 2 , Chuncheng Chen 1, 2 , Jincai Zhao 1, 2
Affiliation
|
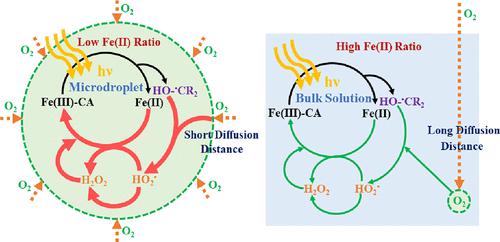
Fe(III) and carboxylic acids are common compositions in atmospheric microdroplet systems like clouds, fogs, and aerosols. Although photochemical processes of Fe(III)–carboxylate complexes have been extensively studied in bulk aqueous solution, relevant information on the dynamic microdroplet system, which may be largely different from the bulk phase, is rare. With the help of the custom-made ultrasonic-based dynamic microdroplet photochemical system, this study examines the photochemical process of Fe(III)–citric acid complexes in microdroplets for the first time. We find that when the degradation extent of citric acid is similar between the microdroplet system and the bulk solution, the significantly lower Fe(II) ratio is present in microdroplet samples due to the rapider reoxidation of photogenerated Fe(II). However, by replacing citric acid with benzoic acid, no much difference in the Fe(II) ratio between microdroplets and bulk solution is observed, which indicates distinct reoxidation pathways of Fe(II). Moreover, the presence of •OH scavenger, namely, methanol, greatly accelerates the reoxidation of photogenerated Fe(II) in both citric acid and benzoic acid situations. Further experiments reveal that the high availability of O2 and the citric acid- or methanol-derived carbon-centered radicals are responsible for the rapider reoxidation of Fe(II) in iron–citric acid microdroplets by prolonging the length of HO2•- and H2O2-involved radical reaction chains. The results in this study may provide a new understanding about iron–citric acid photochemistry in atmospheric liquid particles, which can further influence the photoactivity of particles and the formation of secondary organic aerosols.
中文翻译:

铁-柠檬酸光化学过程中微滴中 Fe(II)/Fe(III) 的快速氧化还原循环
Fe(III) 和羧酸是大气微滴系统(如云、雾和气溶胶)中的常见成分。尽管 Fe(III)-羧酸盐络合物的光化学过程已在本体水溶液中得到广泛研究,但关于可能与本体相有很大不同的动态微滴系统的相关信息很少。借助定制的基于超声波的动态微滴光化学系统,本研究首次检测了微滴中 Fe(III)-柠檬酸络合物的光化学过程。我们发现,当柠檬酸的降解程度在微滴系统和本体溶液之间相似时,由于光生 Fe(II) 的再氧化速度更快,微滴样品中的 Fe(II) 比率显着降低。然而,通过用苯甲酸代替柠檬酸,观察到微滴和本体溶液之间的 Fe(II) 比率没有太大差异,这表明 Fe(II) 的再氧化途径不同。此外,存在• OH 清除剂,即甲醇,在柠檬酸和苯甲酸情况下极大地加速光生Fe(II) 的再氧化。进一步的实验表明,O 2的高可用性和柠檬酸或甲醇衍生的碳中心自由基通过延长 HO 2 • -和H 2 O 2 -参与的自由基反应链。这项研究的结果可能会为大气液体颗粒中的铁-柠檬酸光化学提供新的认识,这可以进一步影响颗粒的光活性和二次有机气溶胶的形成。
更新日期:2023-03-08
中文翻译:

铁-柠檬酸光化学过程中微滴中 Fe(II)/Fe(III) 的快速氧化还原循环
Fe(III) 和羧酸是大气微滴系统(如云、雾和气溶胶)中的常见成分。尽管 Fe(III)-羧酸盐络合物的光化学过程已在本体水溶液中得到广泛研究,但关于可能与本体相有很大不同的动态微滴系统的相关信息很少。借助定制的基于超声波的动态微滴光化学系统,本研究首次检测了微滴中 Fe(III)-柠檬酸络合物的光化学过程。我们发现,当柠檬酸的降解程度在微滴系统和本体溶液之间相似时,由于光生 Fe(II) 的再氧化速度更快,微滴样品中的 Fe(II) 比率显着降低。然而,通过用苯甲酸代替柠檬酸,观察到微滴和本体溶液之间的 Fe(II) 比率没有太大差异,这表明 Fe(II) 的再氧化途径不同。此外,存在• OH 清除剂,即甲醇,在柠檬酸和苯甲酸情况下极大地加速光生Fe(II) 的再氧化。进一步的实验表明,O 2的高可用性和柠檬酸或甲醇衍生的碳中心自由基通过延长 HO 2 • -和H 2 O 2 -参与的自由基反应链。这项研究的结果可能会为大气液体颗粒中的铁-柠檬酸光化学提供新的认识,这可以进一步影响颗粒的光活性和二次有机气溶胶的形成。













































 京公网安备 11010802027423号
京公网安备 11010802027423号