Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2023-03-07 , DOI: 10.1016/j.molstruc.2023.135313 Narender Addu , Hinuja Miriyala , Ravikumar Kapavarapu , Sunder Kumar Kolli , Manojit Pal
|
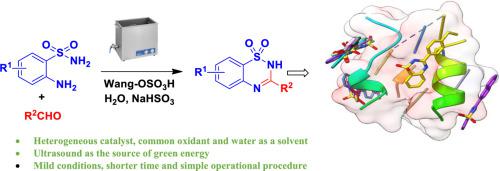
The sonochemical synthesis, in silico assessment and in vitro MtbCM inhibitory activities of a series of 1,2,4-benzothiadiazine-1,1-dioxide derivatives are described. These compounds were synthesized via a one-pot two-step sonochemical method involving the Wang-OSO3H catalyzed reaction of 2-aminobenzenesulfonamide with aldehydes followed by treatment with NaHSO3 in the same pot. The reaction proceeded at room temp in pure water affording the desired products in good yields. The use of heterogeneous catalyst, common oxidant, water as a solvent and ultrasound as the source of green energy in addition to the mild conditions, shorter reaction time and simple operational procedure are the key features of this methodology. In silico studies suggested that most of the synthesized compounds interacted with the external surface pockets of the MtbCM (PDB: 2FP2) active site cavity. Indeed, a curved loop site was noted where these compounds were binding and aligned. Three compounds e.g. 3c, 3d and 3e interacted well with MtbCM via the -NHSO2- moiety of their 2H-benzo[e][1,2,4]thiadiazine-1,1-dioxide ring showing a common H-bond with SER70. They also showed good (55–63%) inhibition of MtbCM in vitro when tested at 10 µM. According to the SAR study a 4-substututed phenyl ring was preferred over a 3- or 2-substutited phenyl moiety at the C-2 position and the 4-MeOC6H4 substituent at this position was most effective in terms of activity. On the other hand, mediocre to low activity was observed when a heteroaryl ring or the bulky 2-naphthyl moiety was present at the C-2 position. Based on in silico and in vitro studies along with the ADME predictions the 1,2,4-benzothiadiazine-1,1-dioxide derivatives 3c, 3d and 3e emerged as pre-hits for further pharmacological evaluations.
中文翻译:

Wang-OSO3H 催化的 1,2,4-苯并噻二嗪-1,1-二氧化物衍生物的一锅声化学合成:它们对 MtbCM 的计算机/体外评估
描述了一系列 1,2,4-苯并噻二嗪-1,1-二氧化物衍生物的声化学合成、计算机评估和体外Mtb CM 抑制活性。这些化合物是通过一锅两步声化学方法合成的,该方法涉及 Wang-OSO 3 H 催化 2-氨基苯磺酰胺与醛的反应,然后用 NaHSO 3处理在同一个锅里。反应在室温下在纯水中进行,以良好的产率提供所需产物。使用非均相催化剂、普通氧化剂、水作为溶剂和超声波作为绿色能源以及温和的条件、较短的反应时间和简单的操作程序是该方法的主要特点。计算机研究表明,大多数合成化合物与Mtb CM(PDB:2FP2)活性位点腔的外表面袋相互作用。事实上,在这些化合物结合和对齐的地方注意到了一个弯曲的环位点。三种化合物如3c 、3d和3e通过-NHSO 2 - 它们的 2 H -benzo[ e ][1,2,4]thiadiazine-1,1-dioxide 环的部分显示出与 SER70 的共同 H 键。当以 10 µM 测试时,它们在体外也显示出良好 (55–63%) 的Mtb CM 抑制作用。根据 SAR 研究,4-取代的苯环优于 C-2 位的 3-或 2-取代的苯基部分,并且该位置的 4-MeOC 6 H 4 取代基在活性方面最有效。另一方面,当杂芳基环或庞大的 2-萘基部分存在于 C-2 位置时,观察到中等至低活性。基于电子计算机体外研究以及 ADME 预测 1,2,4-苯并噻二嗪-1,1-二氧化物衍生物3c、3d和3e作为进一步药理学评估的预命中物出现。






























 京公网安备 11010802027423号
京公网安备 11010802027423号