当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Kilogram-Scale Synthesis of a Key Ulevostinag Subunit Part I: Accessing a Keto-Nucleoside Intermediate from Guanosine
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-03-07 , DOI: 10.1021/acs.oprd.2c00397 Ben W. H. Turnbull 1 , Feng Peng 1 , Andrew J. Neel 1 , Tamas Benkovics 1 , Zhuqing Liu 1 , Cheol K. Chung 1 , Zhiguo Jake Song 1 , Lushi Tan 1 , Khateeta M. Emerson 1 , Chengqian Xiao 2 , Yi Zhang 2 , Benjamin D. Sherry 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-03-07 , DOI: 10.1021/acs.oprd.2c00397 Ben W. H. Turnbull 1 , Feng Peng 1 , Andrew J. Neel 1 , Tamas Benkovics 1 , Zhuqing Liu 1 , Cheol K. Chung 1 , Zhiguo Jake Song 1 , Lushi Tan 1 , Khateeta M. Emerson 1 , Chengqian Xiao 2 , Yi Zhang 2 , Benjamin D. Sherry 1
Affiliation
|
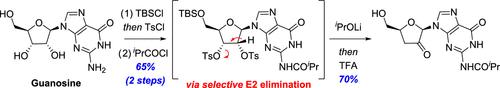
A kilogram-scale synthesis of a key fragment of Ulevostinag (MK-1454), a cyclic dinucleotide agonist of the stimulator of interferon genes (STING), is described. Ulevostinag comprises two non-natural nucleoside derivatives linked together via two P-chiral phosphorothioate groups. The strategy utilized to prepare one of these nucleosides, namely, 3′-deoxy-3′-α-fluoro-guanosine (3′-FG), hinges on a diastereoselective α-fluorination of a key keto-nucleoside derivative, followed by substrate-directed reduction of the ketone. Herein, we describe the development of a robust and scalable synthesis of this intermediate, a 3′-deoxy-2′-keto-guanosine derivative, from guanosine. Salient features of the approach include activation of the 2′ and 3′-alcohol groups of guanosine as a bis-tosylate, which enables regioselective E2 elimination to simultaneously deoxygenate the 3′-position and generate the 2′-ketone.
中文翻译:

关键 Ulevostinag 亚基的千克级合成的发展第 I 部分:从鸟苷中获取酮核苷中间体
描述了 Ulevostinag (MK-1454) 关键片段的千克级合成,Ulevostinag 是干扰素基因 (STING) 刺激物的环状二核苷酸激动剂。Ulevostinag 包含通过两个P连接在一起的两个非天然核苷衍生物-手性硫代磷酸酯基团。用于制备这些核苷之一的策略,即 3'-deoxy-3'-α-fluoro-guanosine (3'-FG),取决于关键酮-核苷衍生物的非对映选择性 α-氟化,然后是底物-定向还原酮。在此,我们描述了这种中间体(一种来自鸟苷的 3'-脱氧-2'-酮-鸟苷衍生物)稳健且可扩展合成的开发。该方法的显着特征包括将鸟苷的 2' 和 3'-醇基激活为双甲苯磺酸盐,这使得区域选择性 E2 消除能够同时使 3'-位脱氧并产生 2'-酮。
更新日期:2023-03-07
中文翻译:

关键 Ulevostinag 亚基的千克级合成的发展第 I 部分:从鸟苷中获取酮核苷中间体
描述了 Ulevostinag (MK-1454) 关键片段的千克级合成,Ulevostinag 是干扰素基因 (STING) 刺激物的环状二核苷酸激动剂。Ulevostinag 包含通过两个P连接在一起的两个非天然核苷衍生物-手性硫代磷酸酯基团。用于制备这些核苷之一的策略,即 3'-deoxy-3'-α-fluoro-guanosine (3'-FG),取决于关键酮-核苷衍生物的非对映选择性 α-氟化,然后是底物-定向还原酮。在此,我们描述了这种中间体(一种来自鸟苷的 3'-脱氧-2'-酮-鸟苷衍生物)稳健且可扩展合成的开发。该方法的显着特征包括将鸟苷的 2' 和 3'-醇基激活为双甲苯磺酸盐,这使得区域选择性 E2 消除能够同时使 3'-位脱氧并产生 2'-酮。






























 京公网安备 11010802027423号
京公网安备 11010802027423号