当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A stepwise oxidation strategy for the synthesis of amorphous V2O5@V2CTx nanohybrid cathodes toward high-performance aqueous Zn-ion batteries
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2023-03-07 , DOI: 10.1039/d2ta09979a Weiwei Wang 1 , Ruiting Hu 1 , Chi Zhang 1 , Yu Tao 1 , Ling Ran 1 , Yani Li 1 , Yao Ouyang 2 , Jun Yan 1, 3, 4
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2023-03-07 , DOI: 10.1039/d2ta09979a Weiwei Wang 1 , Ruiting Hu 1 , Chi Zhang 1 , Yu Tao 1 , Ling Ran 1 , Yani Li 1 , Yao Ouyang 2 , Jun Yan 1, 3, 4
Affiliation
|
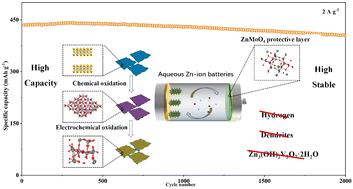
Vanadium-based materials are a potential class of cathode materials for aqueous Zn-ion batteries (ZIBs). However, the low intrinsic conductivity, sluggish kinetics, and poor cycling stability are the key factors hindering their further application. Herein, we report a high-capacity and stable ZIB system based on an amorphous V2O5@V2CTx cathode and Zn@ZnMoO4 anode. The amorphous V2O5@V2CTx cathode was achieved by chemical oxidation of V2CTx in a CO2 atmosphere and subsequent in situ electrochemical oxidation at high potential (∼1.5–1.8 V vs. Zn/Zn2+) in a living cell. First-principles calculations indicate that the low Zn2+ diffusion energy barrier in amorphous V2O5 is the key factor for acquiring high Zn-storage performance. And the continuous formation of Zn3(OH)2V2O7·2H2O (ZVO) with a high Zn2+ diffusion energy barrier on the cathode surface during the charging and discharging process is the culprit for the capacity decay. To ensure stable Zn-storage performance, the formation mechanism of the ZVO was investigated through various ex situ analyses. It was verified that the corrosion reaction of the bare Zn anode promoted the formation of ZVO. By optimizing the bare Zn anode to a reported hydrogen-suppressed Zn@ZnMoO4 anode, the assembled amorphous V2O5@V2CTx//Zn@ZnMoO4 battery can simultaneously achieve high capacity (643.6 mA h g−1 at 0.1 A g−1), excellent rate performance (302.4 mA h g−1 at 20 A g−1) and outstanding cycle stability. In addition, there is also solid evidence confirming the conventional co-intercalation/de-intercalation charge storage mechanism of H+ and Zn2+ in the amorphous V2O5@V2CTx cathode. This work not only reports a high-performance ZIB cathode material but also provides an explanation for the capacity decay of vanadium-based materials during cycling.
中文翻译:

一种用于合成非晶态 V2O5@V2CTx 纳米杂化阴极的逐步氧化策略,用于高性能水性锌离子电池
钒基材料是一类潜在的水系锌离子电池 (ZIB) 正极材料。然而,本征电导率低、动力学缓慢和循环稳定性差是阻碍其进一步应用的关键因素。在此,我们报告了一种基于非晶 V 2 O 5 @V 2 CT x阴极和 Zn@ZnMoO 4阳极的高容量且稳定的 ZIB 系统。无定形 V 2 O 5 @V 2 CT x阴极是通过 V 2 CT x在 CO 2气氛中的化学氧化和随后的原位获得的活细胞中高电位(~1.5–1.8 V vs. Zn/Zn 2+ )下的电化学氧化。第一性原理计算表明,非晶态V 2 O 5中较低的Zn 2+扩散能垒是获得高储锌性能的关键因素。并连续形成高Zn 2+的Zn 3 (OH) 2 V 2 O 7 ·2H 2 O (ZVO)充放电过程中正极表面的扩散能垒是容量衰减的罪魁祸首。为了确保稳定的储锌性能,通过各种非原位分析研究了 ZVO 的形成机制。证实了裸锌阳极的腐蚀反应促进了 ZVO 的形成。通过将裸锌负极优化为已报道的氢抑制 Zn@ZnMoO 4负极,组装的非晶 V 2 O 5 @V 2 CT x //Zn@ZnMoO 4电池可同时实现高容量(643.6 mA hg -1在 0.1 A g −1 ),优异的倍率性能(302.4 mA hg-1在 20 A g -1 ) 和出色的循环稳定性。此外,还有确凿的证据证实非晶态V 2 O 5 @V 2 CT x阴极中H +和Zn 2+的常规共嵌入/脱嵌电荷存储机制。这项工作不仅报道了一种高性能 ZIB 正极材料,而且还解释了钒基材料在循环过程中的容量衰减。
更新日期:2023-03-07
中文翻译:

一种用于合成非晶态 V2O5@V2CTx 纳米杂化阴极的逐步氧化策略,用于高性能水性锌离子电池
钒基材料是一类潜在的水系锌离子电池 (ZIB) 正极材料。然而,本征电导率低、动力学缓慢和循环稳定性差是阻碍其进一步应用的关键因素。在此,我们报告了一种基于非晶 V 2 O 5 @V 2 CT x阴极和 Zn@ZnMoO 4阳极的高容量且稳定的 ZIB 系统。无定形 V 2 O 5 @V 2 CT x阴极是通过 V 2 CT x在 CO 2气氛中的化学氧化和随后的原位获得的活细胞中高电位(~1.5–1.8 V vs. Zn/Zn 2+ )下的电化学氧化。第一性原理计算表明,非晶态V 2 O 5中较低的Zn 2+扩散能垒是获得高储锌性能的关键因素。并连续形成高Zn 2+的Zn 3 (OH) 2 V 2 O 7 ·2H 2 O (ZVO)充放电过程中正极表面的扩散能垒是容量衰减的罪魁祸首。为了确保稳定的储锌性能,通过各种非原位分析研究了 ZVO 的形成机制。证实了裸锌阳极的腐蚀反应促进了 ZVO 的形成。通过将裸锌负极优化为已报道的氢抑制 Zn@ZnMoO 4负极,组装的非晶 V 2 O 5 @V 2 CT x //Zn@ZnMoO 4电池可同时实现高容量(643.6 mA hg -1在 0.1 A g −1 ),优异的倍率性能(302.4 mA hg-1在 20 A g -1 ) 和出色的循环稳定性。此外,还有确凿的证据证实非晶态V 2 O 5 @V 2 CT x阴极中H +和Zn 2+的常规共嵌入/脱嵌电荷存储机制。这项工作不仅报道了一种高性能 ZIB 正极材料,而且还解释了钒基材料在循环过程中的容量衰减。































 京公网安备 11010802027423号
京公网安备 11010802027423号