当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Divergent Synthesis of 3-Pyrrolidin-2-yl-1H-indoles, Symmetric and Unsymmetric Bis(Indolyl)Methanes (BIMs) through Photocatalyzed Decarboxylative Coupling/Friedel–Crafts Alkylation Reaction
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-03-07 , DOI: 10.1021/acs.joc.2c02166 Patamawadee Silalai 1 , Rungnapha Saeeng 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-03-07 , DOI: 10.1021/acs.joc.2c02166 Patamawadee Silalai 1 , Rungnapha Saeeng 1
Affiliation
|
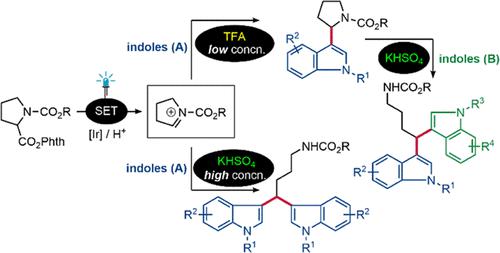
This paper reports the acid-controlled divergent synthesis of 3-pyrrolidin-2-yl-1H-indoles and symmetric and unsymmetrical bis(indolyl)methanes (BIMs) through photocatalyzed decarboxylative coupling and Friedel–Crafts alkylation reactions, respectively. The protocol involves C–H functionalization, switching formation of two products, room-temperature conditions, low photocatalyst loadings, without strong oxidant, and moderate to excellent yields. This method has been applied for the synthesis of natural product vibrindole A and 1,1-bis(1H-indol-3-yl)-2-phenylethane.
中文翻译:

3-Pyrrolidin-2-yl-1H-indoles、对称和不对称双(吲哚基)甲烷 (BIMs) 通过光催化脱羧偶联/Friedel-Crafts 烷基化反应的发散合成
本文分别报道了通过光催化脱羧偶联和 Friedel-Crafts 烷基化反应,酸控发散合成 3-pyrrolidin-2-yl-1 H-吲哚和对称和不对称双(吲哚基)甲烷 (BIM)。该协议涉及 C-H 功能化、两种产品的转换形成、室温条件、低光催化剂负载、无强氧化剂,以及中等到极好的收率。该方法已应用于天然产物vibrindole A和1,1-bis(1 H -indol-3-yl)-2-phenylethane的合成。
更新日期:2023-03-07
中文翻译:

3-Pyrrolidin-2-yl-1H-indoles、对称和不对称双(吲哚基)甲烷 (BIMs) 通过光催化脱羧偶联/Friedel-Crafts 烷基化反应的发散合成
本文分别报道了通过光催化脱羧偶联和 Friedel-Crafts 烷基化反应,酸控发散合成 3-pyrrolidin-2-yl-1 H-吲哚和对称和不对称双(吲哚基)甲烷 (BIM)。该协议涉及 C-H 功能化、两种产品的转换形成、室温条件、低光催化剂负载、无强氧化剂,以及中等到极好的收率。该方法已应用于天然产物vibrindole A和1,1-bis(1 H -indol-3-yl)-2-phenylethane的合成。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号