背景
MK-0616 是一种口服大环肽前蛋白转化酶枯草杆菌蛋白酶/kexin 9 型 (PCSK9) 抑制剂,正在开发用于治疗高胆固醇血症。
目标
这项 2b 期、随机、双盲、安慰剂对照、多中心试验旨在评估 MK-0616 对高胆固醇血症参与者的疗效和安全性。
方法
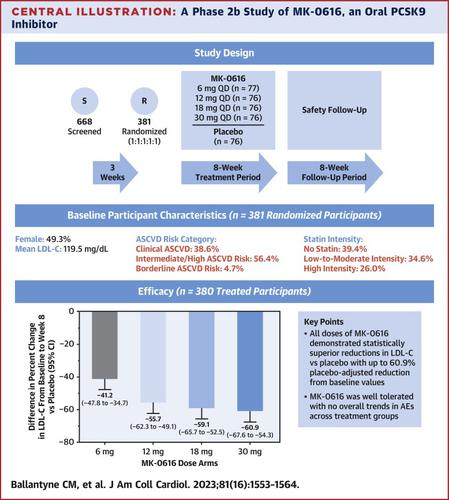
该试验计划纳入 375 名患有多种动脉粥样硬化性心血管疾病风险的成年参与者。参与者被随机分配(1:1:1:1:1 比例)接受 MK-0616(6、12、18 或 30 毫克,每天一次)或匹配的安慰剂。主要终点包括第 8 周时低密度脂蛋白胆固醇 (LDL-C) 相对于基线的百分比变化,以及出现不良事件 (AE) 和因 AE 导致研究干预中断的参与者比例;在 8 周治疗期后,对参与者进行额外 8 周的 AE 监测。
结果
在 381 名随机参与者中,49% 是女性,中位年龄为 62 岁。在 380 名接受治疗的参与者中,与安慰剂相比,所有剂量的 MK-0616 的 LDL-C 从基线到第 8 周的最小二乘平均百分比变化均表现出统计学显着性差异 ( P < 0.001):–41.2% (6 mg)、–55.7% (6 mg) 12 毫克)、–59.1%(18 毫克)和–60.9%(30 毫克)。MK-0616 组参与者中发生 AE 的比例(39.5% 至 43.4%)与安慰剂组(44.0%)相似。在任何治疗组中,因 AE 导致的中止发生在 2 名或更少的参与者中。
结论
MK-0616 在第 8 周表现出统计学上显着且稳健的剂量依赖性安慰剂调整 LDL-C 降低,较基线降低高达 60.9%,并且在 8 周的治疗和另外 8 周的随访期间具有良好的耐受性。(MK-0616 [口服 PCSK9 抑制剂] 对成人高胆固醇血症的疗效和安全性研究 [MK-0616-008];NCT05261126)
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Phase 2b Randomized Trial of the Oral PCSK9 Inhibitor MK-0616
Background
MK-0616 is an oral macrocyclic peptide inhibitor of proprotein convertase subtilisin/kexin type 9 (PCSK9) in development for the treatment of hypercholesterolemia.
Objectives
This Phase 2b, randomized, double-blind, placebo-controlled, multicenter trial aimed to evaluate the efficacy and safety of MK-0616 in participants with hypercholesterolemia.
Methods
This trial was planned to include 375 adult participants with a wide range of atherosclerotic cardiovascular disease risk. Participants were assigned randomly (1:1:1:1:1 ratio) to MK-0616 (6, 12, 18, or 30 mg once daily) or matching placebo. The primary endpoints included percentage change from baseline in low-density lipoprotein cholesterol (LDL-C) at Week 8 and the proportion of participants with adverse events (AEs) and study intervention discontinuations due to AEs; participants were monitored for AEs for an additional 8 weeks after the 8-week treatment period.
Results
Of the 381 participants randomized, 49% were female, and the median age was 62 years. Among 380 treated participants, all doses of MK-0616 demonstrated statistically significant (P < 0.001) differences in least squares mean percentage change in LDL-C from baseline to Week 8 vs placebo: –41.2% (6 mg), –55.7% (12 mg), –59.1% (18 mg), and –60.9% (30 mg). AEs occurred in a similar proportion of participants in the MK-0616 arms (39.5% to 43.4%) as placebo (44.0%). Discontinuations due to AEs occurred in 2 or fewer participants in any treatment group.
Conclusions
MK-0616 demonstrated statistically significant and robust, dose-dependent placebo-adjusted reductions in LDL-C at Week 8 of up to 60.9% from baseline and was well tolerated during 8 weeks of treatment and an additional 8 weeks of follow-up. (A Study of the Efficacy and Safety of MK-0616 [Oral PCSK9 Inhibitor] in Adults With Hypercholesterolemia [MK-0616-008]; NCT05261126)


































 京公网安备 11010802027423号
京公网安备 11010802027423号