当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alloying of Cu with Ru Enabling the Relay Catalysis for Reduction of Nitrate to Ammonia
Advanced Materials ( IF 27.4 ) Pub Date : 2023-03-05 , DOI: 10.1002/adma.202202952 Wensheng Gao 1 , Kefeng Xie 2 , Jin Xie 1 , Xiaomei Wang 1 , Hong Zhang 3 , Shengqi Chen 1 , Hao Wang 1 , Zelong Li 1 , Can Li 1, 4
Advanced Materials ( IF 27.4 ) Pub Date : 2023-03-05 , DOI: 10.1002/adma.202202952 Wensheng Gao 1 , Kefeng Xie 2 , Jin Xie 1 , Xiaomei Wang 1 , Hong Zhang 3 , Shengqi Chen 1 , Hao Wang 1 , Zelong Li 1 , Can Li 1, 4
Affiliation
|
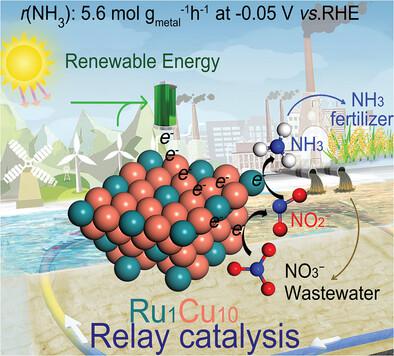
Involving eight electron transfer process and multiple intermediates of nitrate (NO3−) reduction reaction leads to a sluggish kinetic and low Faradaic efficiency, therefore, it is essential to get an insight into the reaction mechanism to develop highly efficient electrocatalyst. Herein, a series of reduced-graphene-oxide-supported RuCu alloy catalysts (RuxCux/rGO) are fabricated and used for the direct reduction of NO3− to NH3. It is found that the Ru1Cu10/rGO shows the ammonia formation rate of 0.38 mmol cm−2 h−1 (loading 1 mg cm−2) and the ammonia Faradaic efficiency of 98% under an ultralow potential of −0.05 V versus Reversible Hydrogen Electode (RHE), which is comparable to Ru catalyst. The highly efficient activity of Ru1Cu10/rGO can be attributed to the synergetic effect between Ru and Cu sites via a relay catalysis, in which the Cu shows the exclusively efficient activity for the reduction of NO3− to NO2− and Ru exhibits the superior activity for NO2− to NH3. In addition, the doping of Ru into Cu tunes the d-band center of alloy and effectively modulates the adsorption energy of the NO3− and NO2−, which promotes the direct reduction of NO3− to NH3. This synergetic electrocatalysis strategy opens a new avenue for developing highly efficient multifunctional catalysts.
中文翻译:

Cu与Ru合金化实现硝酸盐还原为氨的中继催化
涉及八个电子转移过程和硝酸盐(NO 3 - )还原反应的多个中间体导致动力学缓慢和法拉第效率低,因此,必须深入了解反应机理以开发高效电催化剂。在此,制备了一系列还原氧化石墨烯负载的 RuCu 合金催化剂 (Ru x Cu x /rGO),并将其用于将 NO 3 -直接还原为 NH 3。结果发现,Ru 1 Cu 10 /rGO 的氨形成速率为 0.38 mmol cm -2 h -1(加载 1 mg cm -2) 以及在-0.05 V 的超低电位下与可逆氢电极 (RHE) 相比氨法拉第效率为 98%,这与 Ru 催化剂相当。Ru 1 Cu 10 /rGO的高效活性可归因于 Ru 和 Cu 位点之间通过中继催化的协同作用,其中 Cu 显示出将 NO 3 - 还原为NO 2 -和Ru的唯一有效活性对 NO 2 −和 NH 3表现出优异的活性。此外,Cu掺杂Ru调节了合金的d带中心,有效调节了NO 3 -和NO 2 -的吸附能。,这促进了 NO 3 -直接还原为 NH 3。这种协同电催化策略为开发高效多功能催化剂开辟了一条新途径。
更新日期:2023-03-05
中文翻译:

Cu与Ru合金化实现硝酸盐还原为氨的中继催化
涉及八个电子转移过程和硝酸盐(NO 3 - )还原反应的多个中间体导致动力学缓慢和法拉第效率低,因此,必须深入了解反应机理以开发高效电催化剂。在此,制备了一系列还原氧化石墨烯负载的 RuCu 合金催化剂 (Ru x Cu x /rGO),并将其用于将 NO 3 -直接还原为 NH 3。结果发现,Ru 1 Cu 10 /rGO 的氨形成速率为 0.38 mmol cm -2 h -1(加载 1 mg cm -2) 以及在-0.05 V 的超低电位下与可逆氢电极 (RHE) 相比氨法拉第效率为 98%,这与 Ru 催化剂相当。Ru 1 Cu 10 /rGO的高效活性可归因于 Ru 和 Cu 位点之间通过中继催化的协同作用,其中 Cu 显示出将 NO 3 - 还原为NO 2 -和Ru的唯一有效活性对 NO 2 −和 NH 3表现出优异的活性。此外,Cu掺杂Ru调节了合金的d带中心,有效调节了NO 3 -和NO 2 -的吸附能。,这促进了 NO 3 -直接还原为 NH 3。这种协同电催化策略为开发高效多功能催化剂开辟了一条新途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号