当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible Light Photoredox-Catalyzed Decarboxylative Alkylation of 3-Aryl-Oxetanes and Azetidines via Benzylic Tertiary Radicals and Implications of Benzylic Radical Stability
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-03-03 , DOI: 10.1021/acs.joc.3c00083 Maryne A J Dubois 1 , Juan J Rojas 1 , Alistair J Sterling 2 , Hannah C Broderick 1 , Milo A Smith 1 , Andrew J P White 1 , Philip W Miller 1 , Chulho Choi 3 , James J Mousseau 3 , Fernanda Duarte 2 , James A Bull 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-03-03 , DOI: 10.1021/acs.joc.3c00083 Maryne A J Dubois 1 , Juan J Rojas 1 , Alistair J Sterling 2 , Hannah C Broderick 1 , Milo A Smith 1 , Andrew J P White 1 , Philip W Miller 1 , Chulho Choi 3 , James J Mousseau 3 , Fernanda Duarte 2 , James A Bull 1
Affiliation
|
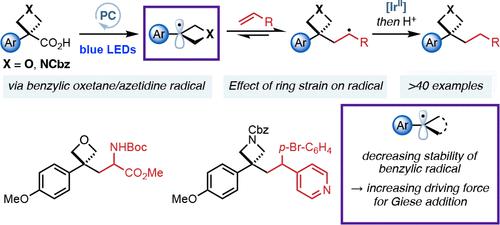
Four-membered heterocycles offer exciting potential as small polar motifs in medicinal chemistry but require further methods for incorporation. Photoredox catalysis is a powerful method for the mild generation of alkyl radicals for C–C bond formation. The effect of ring strain on radical reactivity is not well understood, with no studies that address this question systematically. Examples of reactions that involve benzylic radicals are rare, and their reactivity is challenging to harness. This work develops a radical functionalization of benzylic oxetanes and azetidines using visible light photoredox catalysis to prepare 3-aryl-3-alkyl substituted derivatives and assesses the influence of ring strain and heterosubstitution on the reactivity of small-ring radicals. 3-Aryl-3-carboxylic acid oxetanes and azetidines are suitable precursors to tertiary benzylic oxetane/azetidine radicals which undergo conjugate addition into activated alkenes. We compare the reactivity of oxetane radicals to other benzylic systems. Computational studies indicate that Giese additions of unstrained benzylic radicals into acrylates are reversible and result in low yields and radical dimerization. Benzylic radicals as part of a strained ring, however, are less stable and more π-delocalized, decreasing dimer and increasing Giese product formation. Oxetanes show high product yields due to ring strain and Bent’s rule rendering the Giese addition irreversible.
中文翻译:

可见光光氧化还原催化 3-芳基-氧杂环丁烷和氮杂环丁烷通过苄基叔自由基的脱羧烷基化和苄基自由基稳定性的意义
四元杂环作为药物化学中的小极性基序具有令人兴奋的潜力,但需要进一步的掺入方法。光氧化还原催化是温和生成用于 C-C 键形成的烷基自由基的有效方法。环应变对自由基反应性的影响尚不清楚,没有系统地解决这个问题的研究。涉及苄基的反应例子很少见,而且它们的反应性很难驾驭。这项工作使用可见光光氧化还原催化开发了苄基氧杂环丁烷和氮杂环丁烷的自由基官能化,以制备 3-aryl-3-alkyl 取代的衍生物,并评估了环应变和杂取代对小环自由基反应性的影响。3-芳基-3-羧酸氧杂环丁烷和氮杂环丁烷是叔苄基氧杂环丁烷/氮杂环丁烷自由基的合适前体,它们经历共轭加成到活化的烯烃中。我们比较了氧杂环丁烷自由基与其他苄基系统的反应性。计算研究表明,将未应变的苄基自由基 Giese 加成到丙烯酸酯中是可逆的,并导致低产率和自由基二聚化。然而,作为应变环的一部分的苄基自由基稳定性较差且 π 离域更多,从而减少二聚体并增加 Giese 产物的形成。氧杂环丁烷由于环应变和使 Giese 加成不可逆的 Bent 规则显示出高产率。计算研究表明,将未应变的苄基自由基 Giese 加成到丙烯酸酯中是可逆的,并导致低产率和自由基二聚化。然而,作为应变环的一部分的苄基自由基稳定性较差且 π 离域较多,从而减少二聚体并增加 Giese 产物的形成。氧杂环丁烷由于环应变和使 Giese 加成不可逆的 Bent 规则显示出高产率。计算研究表明,将未应变的苄基自由基 Giese 加成到丙烯酸酯中是可逆的,并导致低产率和自由基二聚化。然而,作为应变环的一部分的苄基自由基稳定性较差且 π 离域较多,从而减少二聚体并增加 Giese 产物的形成。氧杂环丁烷由于环应变和使 Giese 加成不可逆的 Bent 规则显示出高产率。
更新日期:2023-03-03
中文翻译:

可见光光氧化还原催化 3-芳基-氧杂环丁烷和氮杂环丁烷通过苄基叔自由基的脱羧烷基化和苄基自由基稳定性的意义
四元杂环作为药物化学中的小极性基序具有令人兴奋的潜力,但需要进一步的掺入方法。光氧化还原催化是温和生成用于 C-C 键形成的烷基自由基的有效方法。环应变对自由基反应性的影响尚不清楚,没有系统地解决这个问题的研究。涉及苄基的反应例子很少见,而且它们的反应性很难驾驭。这项工作使用可见光光氧化还原催化开发了苄基氧杂环丁烷和氮杂环丁烷的自由基官能化,以制备 3-aryl-3-alkyl 取代的衍生物,并评估了环应变和杂取代对小环自由基反应性的影响。3-芳基-3-羧酸氧杂环丁烷和氮杂环丁烷是叔苄基氧杂环丁烷/氮杂环丁烷自由基的合适前体,它们经历共轭加成到活化的烯烃中。我们比较了氧杂环丁烷自由基与其他苄基系统的反应性。计算研究表明,将未应变的苄基自由基 Giese 加成到丙烯酸酯中是可逆的,并导致低产率和自由基二聚化。然而,作为应变环的一部分的苄基自由基稳定性较差且 π 离域更多,从而减少二聚体并增加 Giese 产物的形成。氧杂环丁烷由于环应变和使 Giese 加成不可逆的 Bent 规则显示出高产率。计算研究表明,将未应变的苄基自由基 Giese 加成到丙烯酸酯中是可逆的,并导致低产率和自由基二聚化。然而,作为应变环的一部分的苄基自由基稳定性较差且 π 离域较多,从而减少二聚体并增加 Giese 产物的形成。氧杂环丁烷由于环应变和使 Giese 加成不可逆的 Bent 规则显示出高产率。计算研究表明,将未应变的苄基自由基 Giese 加成到丙烯酸酯中是可逆的,并导致低产率和自由基二聚化。然而,作为应变环的一部分的苄基自由基稳定性较差且 π 离域较多,从而减少二聚体并增加 Giese 产物的形成。氧杂环丁烷由于环应变和使 Giese 加成不可逆的 Bent 规则显示出高产率。

































 京公网安备 11010802027423号
京公网安备 11010802027423号