Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic Understanding of Catalytic Selectivity and Product Distribution of Electrochemical Carbon Dioxide Reduction Reaction
JACS Au ( IF 8.5 ) Pub Date : 2023-03-02 , DOI: 10.1021/jacsau.3c00002 Dai-Jian Su 1 , Shi-Qin Xiang 1 , Shu-Ting Gao 1 , Yimin Jiang 1 , Xiaohong Liu 2 , Wei Zhang 2 , Liu-Bin Zhao 1 , Zhong-Qun Tian 3
JACS Au ( IF 8.5 ) Pub Date : 2023-03-02 , DOI: 10.1021/jacsau.3c00002 Dai-Jian Su 1 , Shi-Qin Xiang 1 , Shu-Ting Gao 1 , Yimin Jiang 1 , Xiaohong Liu 2 , Wei Zhang 2 , Liu-Bin Zhao 1 , Zhong-Qun Tian 3
Affiliation
|
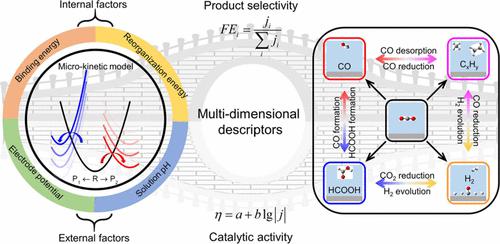
CO2 can be electrochemically reduced to different products depending on the nature of catalysts. In this work, we report comprehensive kinetic studies on catalytic selectivity and product distribution of the CO2 reduction reaction on various metal surfaces. The influences on reaction kinetics can be clearly analyzed from the variation of reaction driving force (binding energy difference) and reaction resistance (reorganization energy). Moreover, the CO2RR product distributions are further affected by external factors such as electrode potential and solution pH. A potential-mediated mechanism is found to determine the competing two-electron reduction products of CO2 that shifts from thermodynamics-controlled product formic acid at less negative electrode potentials to kinetic-controlled product CO at more negative electrode potentials. Based on detailed kinetic simulations, a three-parameter descriptor is applied to identify the catalytic selectivity of CO, formate, hydrocarbons/alcohols, as well as side product H2. The present kinetic study not only well explains the catalytic selectivity and product distribution of experimental results but also provides a fast way for catalyst screening.
中文翻译:

电化学二氧化碳还原反应的催化选择性和产物分布的动力学理解
取决于催化剂的性质,CO 2可以被电化学还原成不同的产物。在这项工作中,我们报告了对各种金属表面上CO 2还原反应的催化选择性和产物分布的综合动力学研究。从反应驱动力(结合能差)和反应阻力(重组能)的变化可以清楚地分析对反应动力学的影响。此外,CO 2 RR 产物的分布还受到电极电位和溶液 pH 值等外部因素的影响。发现了一种电位介导的机制来确定 CO 2的竞争性双电子还原产物从负电极电位较低的热力学控制产物甲酸转变为负电极电位较高的动力学控制产物 CO。基于详细的动力学模拟,应用三参数描述符来确定 CO、甲酸盐、碳氢化合物/醇以及副产物 H 2的催化选择性。本动力学研究不仅很好地解释了实验结果的催化选择性和产物分布,而且为催化剂筛选提供了一种快速途径。
更新日期:2023-03-02
中文翻译:

电化学二氧化碳还原反应的催化选择性和产物分布的动力学理解
取决于催化剂的性质,CO 2可以被电化学还原成不同的产物。在这项工作中,我们报告了对各种金属表面上CO 2还原反应的催化选择性和产物分布的综合动力学研究。从反应驱动力(结合能差)和反应阻力(重组能)的变化可以清楚地分析对反应动力学的影响。此外,CO 2 RR 产物的分布还受到电极电位和溶液 pH 值等外部因素的影响。发现了一种电位介导的机制来确定 CO 2的竞争性双电子还原产物从负电极电位较低的热力学控制产物甲酸转变为负电极电位较高的动力学控制产物 CO。基于详细的动力学模拟,应用三参数描述符来确定 CO、甲酸盐、碳氢化合物/醇以及副产物 H 2的催化选择性。本动力学研究不仅很好地解释了实验结果的催化选择性和产物分布,而且为催化剂筛选提供了一种快速途径。






























 京公网安备 11010802027423号
京公网安备 11010802027423号