当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solution NMR of Battery Electrolytes: Assessing and Mitigating Spectral Broadening Caused by Transition Metal Dissolution
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2023-02-28 , DOI: 10.1021/acs.jpcc.2c08274 Jennifer P Allen 1, 2 , Clare P Grey 1, 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2023-02-28 , DOI: 10.1021/acs.jpcc.2c08274 Jennifer P Allen 1, 2 , Clare P Grey 1, 2
Affiliation
|
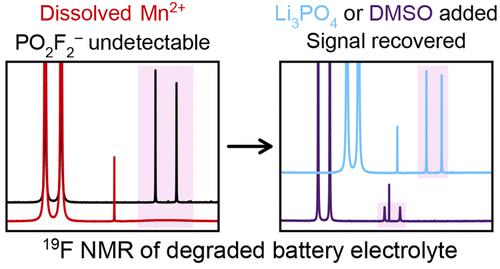
NMR spectroscopy is a powerful tool that is commonly used to assess the degradation of lithium-ion battery electrolyte solutions. However, dissolution of paramagnetic Ni2+ and Mn2+ ions from cathode materials may affect the NMR spectra of the electrolyte solution, with the unpaired electron spins in these paramagnetic solutes inducing rapid nuclear relaxation and spectral broadening (and often peak shifts). This work establishes how dissolved Ni2+ and Mn2+ in LiPF6 electrolyte solutions affect 1H, 19F, and 31P NMR spectra of pristine and degraded electrolyte solutions, including whether the peaks from degradation species are at risk of being lost and whether the spectral broadening can be mitigated. Mn2+ is shown to cause far greater peak broadening than Ni2+, with the effect of Mn2+ observable at just 10 μM. Generally, 19F peaks from PF6– degradation species are most affected by the presence of the paramagnetic metals, followed by 31P and 1H peaks. Surprisingly, when NMR solvents are added to acquire the spectra, the degree of broadening is heavily solvent-dependent, following the trend of solvent donor number (increased broadening with lower solvent donicity). Severe spectral broadening is shown to occur whether Mn is introduced via the salt Mn(TFSI)2 or is dissolved from LiMn2O4. We show that the weak 19F and 31P peaks in spectra of electrolyte samples containing micromolar levels of dissolved Mn2+ are broadened to an extent that they are no longer visible, but this broadening can be minimized by diluting electrolyte samples with a suitably coordinating NMR solvent. Li3PO4 addition to the sample is also shown to return 19F and 31P spectral resolution by precipitating Mn2+ out of electrolyte samples, although this method consumes any HF in the electrolyte solution.
中文翻译:

电池电解质的溶液 NMR:评估和减轻由过渡金属溶解引起的光谱展宽
NMR 光谱是一种强大的工具,通常用于评估锂离子电池电解质溶液的降解。然而,来自阴极材料的顺磁性 Ni 2+和 Mn 2+离子的溶解可能会影响电解质溶液的 NMR 光谱,这些顺磁性溶质中的不成对电子自旋会引起快速核弛豫和光谱展宽(并且通常会出现峰移)。这项工作确定了LiPF 6电解质溶液中溶解的 Ni 2+和 Mn 2+如何影响1 H、19 F 和31原始和降解电解质溶液的 P NMR 光谱,包括降解物质的峰是否有丢失的风险以及光谱展宽是否可以减轻。Mn 2+比 Ni 2+引起的峰展宽大得多,Mn 2+的影响仅在 10 μM 处即可观察到。通常,来自 PF 6的19 F 峰-降解物质受顺磁性金属存在的影响最大,其次是31 P 和1H峰。令人惊讶的是,当添加 NMR 溶剂以获取光谱时,加宽程度在很大程度上取决于溶剂,遵循溶剂供体数量的趋势(增加加宽,溶剂多度性较低)。无论 Mn 是通过盐 Mn(TFSI) 2引入还是从 LiMn 2 O 4中溶解,都表明会发生严重的光谱展宽。我们表明,含有微摩尔水平溶解 Mn 2+的电解质样品光谱中的弱19 F 和31 P 峰被加宽到不再可见的程度,但可以通过使用适当协调的稀释电解质样品来最小化这种加宽核磁共振溶剂。李3 PO 4除了样品之外,还显示通过从电解质样品中沉淀出 Mn 2+来返回19 F 和31 P 光谱分辨率,尽管该方法消耗电解质溶液中的任何 HF。
更新日期:2023-02-28
中文翻译:

电池电解质的溶液 NMR:评估和减轻由过渡金属溶解引起的光谱展宽
NMR 光谱是一种强大的工具,通常用于评估锂离子电池电解质溶液的降解。然而,来自阴极材料的顺磁性 Ni 2+和 Mn 2+离子的溶解可能会影响电解质溶液的 NMR 光谱,这些顺磁性溶质中的不成对电子自旋会引起快速核弛豫和光谱展宽(并且通常会出现峰移)。这项工作确定了LiPF 6电解质溶液中溶解的 Ni 2+和 Mn 2+如何影响1 H、19 F 和31原始和降解电解质溶液的 P NMR 光谱,包括降解物质的峰是否有丢失的风险以及光谱展宽是否可以减轻。Mn 2+比 Ni 2+引起的峰展宽大得多,Mn 2+的影响仅在 10 μM 处即可观察到。通常,来自 PF 6的19 F 峰-降解物质受顺磁性金属存在的影响最大,其次是31 P 和1H峰。令人惊讶的是,当添加 NMR 溶剂以获取光谱时,加宽程度在很大程度上取决于溶剂,遵循溶剂供体数量的趋势(增加加宽,溶剂多度性较低)。无论 Mn 是通过盐 Mn(TFSI) 2引入还是从 LiMn 2 O 4中溶解,都表明会发生严重的光谱展宽。我们表明,含有微摩尔水平溶解 Mn 2+的电解质样品光谱中的弱19 F 和31 P 峰被加宽到不再可见的程度,但可以通过使用适当协调的稀释电解质样品来最小化这种加宽核磁共振溶剂。李3 PO 4除了样品之外,还显示通过从电解质样品中沉淀出 Mn 2+来返回19 F 和31 P 光谱分辨率,尽管该方法消耗电解质溶液中的任何 HF。















































 京公网安备 11010802027423号
京公网安备 11010802027423号