当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Noble-Metal–Metalloid Alloy Architectures: Mesoporous Amorphous Iridium–Tellurium Alloy for Electrochemical N2 Reduction
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-02-28 , DOI: 10.1021/jacs.2c10637
Bo Jiang 1 , Hairong Xue 2 , Pei Wang 3 , Haoran Du 1 , Yunqing Kang 2 , Jingjing Zhao 1 , Shengyao Wang 3 , Wei Zhou 4 , Zhenfeng Bian 1 , Hexing Li 1 , Joel Henzie 2 , Yusuke Yamauchi 5, 6
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-02-28 , DOI: 10.1021/jacs.2c10637
Bo Jiang 1 , Hairong Xue 2 , Pei Wang 3 , Haoran Du 1 , Yunqing Kang 2 , Jingjing Zhao 1 , Shengyao Wang 3 , Wei Zhou 4 , Zhenfeng Bian 1 , Hexing Li 1 , Joel Henzie 2 , Yusuke Yamauchi 5, 6
Affiliation
|
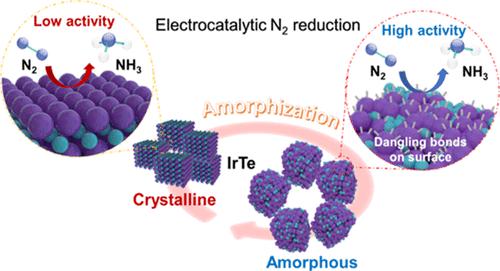
Amorphous noble metals with high surface areas have attracted significant interest as heterogeneous catalysts due to the numerous dangling bonds and abundant unsaturated surface atoms created by the amorphous phase. However, synthesizing amorphous noble metals with high surface areas remains a significant challenge due to strong isotropic metallic bonds. This paper describes the first example of a mesoporous amorphous noble metal alloy [iridium–tellurium (IrTe)] obtained using a micelle-directed synthesis method. The resulting mesoporous amorphous IrTe electrocatalyst exhibits excellent performance in the electrochemical N2 reduction reaction. The ammonia yield rate is 34.6 μg mg–1 h–1 with a Faradaic efficiency of 11.2% at −0.15 V versus reversible hydrogen electrode in 0.1 M HCl solution, outperforming comparable crystalline and Ir metal counterparts. The interconnected porous scaffold and amorphous nature of the alloy create a complementary effect that simultaneously enhances N2 absorption and suppresses the hydrogen evolution reaction. According to theoretical simulations, incorporating Te in the IrTe alloy effectively strengthens the adsorption of N2 and lowers the Gibbs free energy for the rate-limiting step of the electrocatalytic N2 reduction reaction. Mesoporous chemistry enables a new route to achieve high-performance amorphous metalloid alloys with properties that facilitate the selective electrocatalytic reduction of N2.
中文翻译:

贵金属-类金属合金架构:用于电化学 N2 还原的介孔非晶铱-碲合金
具有高表面积的无定形贵金属作为多相催化剂引起了人们的极大兴趣,因为无定形相产生了大量的悬空键和丰富的不饱和表面原子。然而,由于强各向同性金属键,合成具有高表面积的非晶贵金属仍然是一个重大挑战。本文描述了使用胶束定向合成方法获得的介孔非晶贵金属合金 [铱-碲 (IrTe)] 的第一个示例。所得介孔无定形 IrTe 电催化剂在电化学 N 2还原反应中表现出优异的性能。氨产率为 34.6 μg mg –1 h –1在 0.1 M HCl 溶液中与可逆氢电极相比,在 -0.15 V 时的法拉第效率为 11.2%,优于可比较的晶体和 Ir 金属对应物。互连的多孔支架和合金的无定形性质产生互补效应,同时增强 N 2吸收并抑制析氢反应。根据理论模拟,在IrTe合金中加入Te有效地增强了N 2的吸附,降低了电催化N 2还原反应限速步骤的吉布斯自由能。介孔化学为获得高性能非晶准金属合金开辟了一条新途径,该合金具有促进 N 选择性电催化还原的特性2 .
更新日期:2023-02-28
中文翻译:

贵金属-类金属合金架构:用于电化学 N2 还原的介孔非晶铱-碲合金
具有高表面积的无定形贵金属作为多相催化剂引起了人们的极大兴趣,因为无定形相产生了大量的悬空键和丰富的不饱和表面原子。然而,由于强各向同性金属键,合成具有高表面积的非晶贵金属仍然是一个重大挑战。本文描述了使用胶束定向合成方法获得的介孔非晶贵金属合金 [铱-碲 (IrTe)] 的第一个示例。所得介孔无定形 IrTe 电催化剂在电化学 N 2还原反应中表现出优异的性能。氨产率为 34.6 μg mg –1 h –1在 0.1 M HCl 溶液中与可逆氢电极相比,在 -0.15 V 时的法拉第效率为 11.2%,优于可比较的晶体和 Ir 金属对应物。互连的多孔支架和合金的无定形性质产生互补效应,同时增强 N 2吸收并抑制析氢反应。根据理论模拟,在IrTe合金中加入Te有效地增强了N 2的吸附,降低了电催化N 2还原反应限速步骤的吉布斯自由能。介孔化学为获得高性能非晶准金属合金开辟了一条新途径,该合金具有促进 N 选择性电催化还原的特性2 .































 京公网安备 11010802027423号
京公网安备 11010802027423号