Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
对未处理血浆微样品中循环 MicroRNA 进行无扩增、高通量纳米等离子体定量,用于早期胰腺癌检测
ACS Sensors ( IF 8.2 ) Pub Date : 2023-02-28 , DOI: 10.1021/acssensors.2c02105 Adrianna N Masterson 1 , Nayela N Chowdhury 2, 3 , Yue Fang 4 , Michele T Yip-Schneider 5 , Sumon Hati 1 , Prashant Gupta 6 , Sha Cao 4 , Huangbing Wu 5 , C Max Schmidt 3, 5 , Melissa L Fishel 2, 3, 7 , Rajesh Sardar 1, 3
Affiliation
|
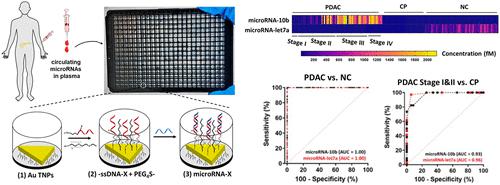
胰腺导管腺癌(PDAC)是一种致命的恶性肿瘤,通常在晚期才被发现。 PDAC 的早期诊断是降低死亡率的关键。 microRNA 等循环生物标志物越来越受到人们的关注,但现有技术需要大量样本、扩增步骤、大量的生物流体处理、缺乏灵敏度且通量低。在这里,我们提出了一种先进的纳米等离子体传感器,用于对未处理的血浆微样品中的 microRNA(microRNA-10b、microRNA-let7a)进行高灵敏度、无扩增检测和定量。该传感器结构利用独特设计的-ssDNA受体附着在金三角纳米棱柱上,以多孔板的形式显示出独特的局域表面等离子共振(LSPR)特性。 −ssDNA/microRNA 双链体的形成控制纳米结构-生物分子界面电子相互作用,以促进电荷转移/激子离域过程并增强局域表面等离子体共振响应,从而在人血浆中实现阿托摩尔 (10 –18 M) 的检测限 (LOD)。这种改进的 LOD 允许以 384 孔板形式进行高通量测定。通过与 15 个 PDAC 患者血浆样本的 qRT-PCR 检测进行比较,进一步评估了纳米等离子体传感器用于 microRNA 检测的性能,结果表明这两种检测之间呈正相关,Pearson 相关系数值 >0.86。对超过 170 个临床样本的评估表明,致癌 microRNA-10b 和肿瘤抑制 microRNA-let7a 水平可以单独区分 PDAC 与慢性胰腺炎和正常对照,灵敏度 >94%,特异性 >94%,置信区间 (CI) 为 95%。 此外,与单个 microRNA 的敏感性和特异性值相比,结合致癌和肿瘤抑制 microRNA 水平可显着改善 PDAC I 期和 II 期与 III 期和 IV 期的分化,灵敏度和特异性分别 >91% 和 87%。此外,我们发现 PDAC 患者术前和术后的 microRNA 水平差异很大( n = 75)。总而言之,这种超灵敏的纳米等离子体传感器具有出色的灵敏度和特异性,能够同时检测多种生物标志物,并可能有助于 PDAC 的早期检测,从而改善患者护理。

"点击查看英文标题和摘要"


















































 京公网安备 11010802027423号
京公网安备 11010802027423号