当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
2-(4-Fluorophenyl)-1H-benzo[d]imidazole as a Promising Template for the Development of Metabolically Robust, α1β2γ2GABA-A Receptor-Positive Allosteric Modulators
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2023-02-27 , DOI: 10.1021/acschemneuro.2c00800 Monika Marcinkowska 1 , Nikola Fajkis-Zajączkowska 1 , Katarzyna Szafrańska 1 , Jakub Jończyk 1 , Agata Siwek 2 , Barbara Mordyl 2 , Tadeusz Karcz 3 , Gniewomir Latacz 3 , Marcin Kolaczkowski 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2023-02-27 , DOI: 10.1021/acschemneuro.2c00800 Monika Marcinkowska 1 , Nikola Fajkis-Zajączkowska 1 , Katarzyna Szafrańska 1 , Jakub Jończyk 1 , Agata Siwek 2 , Barbara Mordyl 2 , Tadeusz Karcz 3 , Gniewomir Latacz 3 , Marcin Kolaczkowski 1
Affiliation
|
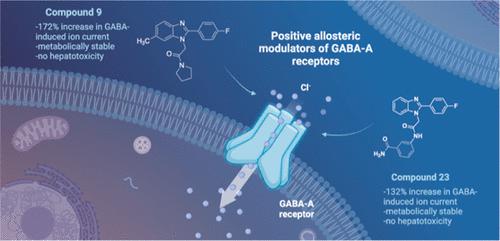
Modulation of α1β2γ2GABA-A receptor subpopulation expressed in the basal ganglia region is a conceptually novel mode of pharmacological strategy that offers prospects to tackle a variety of neurological dysfunction. Although clinical findings provided compelling evidence for the validity of this strategy, the current chemical space of molecules able to modulate the α1/γ2 interface of the GABA-A receptor is limited to imidazo[1,2-a]pyridine derivatives that undergo rapid biotransformation. In response to a deficiency in the chemical repertoire of GABA-A receptors, we identified a series of 2-(4-fluorophenyl)-1H-benzo[d]imidazoles as positive allosteric modulators (PAMs) with improved metabolic stability and reduced potential for hepatotoxicity, where lead molecules 9 and 23 displayed interesting features in a preliminary investigation. We further disclose that the identified scaffold shows a preference for interaction with the α1/γ2 interface of the GABA-A receptor, delivering several PAMs of the GABA-A receptor. The present work provides useful chemical templates to further explore the therapeutic potential of GABA-A receptor ligands and enriches the chemical space of molecules suitable for the interaction with the α1/γ2 interface.
中文翻译:

2-(4-氟苯基)-1H-苯并[d]咪唑作为开发代谢稳健的 α1β2γ2GABA-A 受体正变构调节剂的有前途的模板
调节在基底神经节区域表达的 α1β2γ2GABA-A 受体亚群是一种概念上新颖的药理学策略模式,为解决各种神经功能障碍提供了前景。尽管临床研究结果为该策略的有效性提供了令人信服的证据,但目前能够调节 GABA-A 受体 α1/γ2 界面的分子的化学空间仅限于经历快速生物转化的咪唑并 [1,2-a]吡啶衍生物. 为了应对 GABA-A 受体化学库的缺陷,我们鉴定了一系列 2-(4-氟苯基)-1 H -苯并[ d]咪唑作为正变构调节剂 (PAM),具有改善的代谢稳定性和降低的肝毒性可能性,其中先导分子9和23在初步研究中显示出有趣的特征。我们进一步披露,已识别的支架显示出与 GABA-A 受体的 α1/γ2 界面相互作用的偏好,提供 GABA-A 受体的几种 PAM。目前的工作提供了有用的化学模板,以进一步探索 GABA-A 受体配体的治疗潜力,并丰富了适合与 α1/γ2 界面相互作用的分子的化学空间。
更新日期:2023-02-27
中文翻译:

2-(4-氟苯基)-1H-苯并[d]咪唑作为开发代谢稳健的 α1β2γ2GABA-A 受体正变构调节剂的有前途的模板
调节在基底神经节区域表达的 α1β2γ2GABA-A 受体亚群是一种概念上新颖的药理学策略模式,为解决各种神经功能障碍提供了前景。尽管临床研究结果为该策略的有效性提供了令人信服的证据,但目前能够调节 GABA-A 受体 α1/γ2 界面的分子的化学空间仅限于经历快速生物转化的咪唑并 [1,2-a]吡啶衍生物. 为了应对 GABA-A 受体化学库的缺陷,我们鉴定了一系列 2-(4-氟苯基)-1 H -苯并[ d]咪唑作为正变构调节剂 (PAM),具有改善的代谢稳定性和降低的肝毒性可能性,其中先导分子9和23在初步研究中显示出有趣的特征。我们进一步披露,已识别的支架显示出与 GABA-A 受体的 α1/γ2 界面相互作用的偏好,提供 GABA-A 受体的几种 PAM。目前的工作提供了有用的化学模板,以进一步探索 GABA-A 受体配体的治疗潜力,并丰富了适合与 α1/γ2 界面相互作用的分子的化学空间。






























 京公网安备 11010802027423号
京公网安备 11010802027423号