当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Raman Mapping as a Tool for Evaluating I2 and I3– Diffusion Over Single-Crystal UiO-67_NH2(M) (M = Zr, Zr/Hf, or Hf)
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2023-02-28 , DOI: 10.1021/acs.jpcc.2c08723 Pedro H. M. Andrade 1 , Myriam Moreau 1 , Natacha Henry 2 , Mohamed T. Bakouche 2 , Sylvain Duval 2 , Christophe Volkringer 2 , Thierry Loiseau 2 , Matthieu Hureau 1 , Alain Moissette 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2023-02-28 , DOI: 10.1021/acs.jpcc.2c08723 Pedro H. M. Andrade 1 , Myriam Moreau 1 , Natacha Henry 2 , Mohamed T. Bakouche 2 , Sylvain Duval 2 , Christophe Volkringer 2 , Thierry Loiseau 2 , Matthieu Hureau 1 , Alain Moissette 1
Affiliation
|
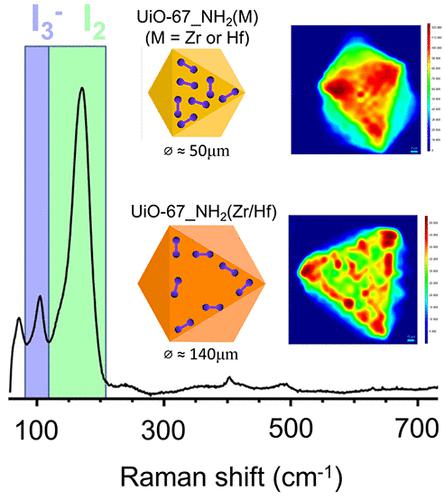
The capture of gaseous iodine has been deeply studied for trying to mitigate the dangers of nuclear power energy. The UiO family of metal–organic framework (MOF) materials is considered as one of the best candidates for such purposes since it couples high specific surface areas, facility to be chemically modified, great iodine adsorption capacity, and good stability under nuclear accidents conditions. UiO-66 was profoundly evaluated in several works for trapping I2 by using different linkers and metal contents. A transformation of the I2 molecule into I3– inside such porous systems was verified in other studies and is yet to be better elucidated. The comprehension of this transformation can improve the materials used to capture iodine species and guarantee a better stabilization of such pollutants in the long term. For this reason, three UiO-67_NH2 samples with different metal contents (Zr, Zr/Hf, and Hf) were employed to capture iodine, and the signature of the different species was evaluated using Raman spectroscopy mappings in and out of resonance conditions (λex = 515, 633, and 785 nm). The UiO-67_NH2(Hf) compound demonstrated the best adsorption capacity after 48 h of contact with gaseous I2 under room temperature, capturing 3428 g·mol–1 of iodine. The other two samples, UiO-67_NH2(Zr/Hf) and UiO-67_NH2(Zr), adsorbed 2835 g·mol–1 and 1658 g·mol–1 in the same conditions, respectively. The I2 transformation into I3– was confirmed by the presence of bands related to “perturbed” I2 and I3– at about 170 and 107 cm–1, respectively. The Raman mapping demonstrated that both the monometallic UiO-67_NH2 samples displayed a homogeneous distribution of the two species after 48 h of contact with the iodine gas flow, whereas the bimetallic sample exhibited zones with different concentrations of I2 and I3–. This effect was related to the I2 diffusion process through the UiO-67_NH2 crystallites, which could be faster in the monometallic UiO-67_NH2 samples because of their smaller crystal size (ϕ ≈ 44 μm and ϕ ≈ 51 μm for UiO-67_NH2(Hf) and UiO-67_NH2(Zr), respectively) when compared to the UiO-67_NH2(Zr/Hf) sample (ϕ ≈ 140 μm). This paper shows the spatial distribution of I2 and I3– along the crystals of UiO-67_NH2 materials and correlates this data with the diffusion process of both species, improving the comprehension of the mechanism responsible for iodine conversion and stabilization in UiO materials.
中文翻译:

拉曼映射作为评估 I2 和 I3 的工具——单晶 UiO-67_NH2(M) 上的扩散(M = Zr、Zr/Hf 或 Hf)
气态碘的捕获已被深入研究以试图减轻核能的危险。UiO 系列金属有机骨架 (MOF) 材料被认为是此类用途的最佳候选材料之一,因为它具有高比表面积、易于化学改性、强大的碘吸附能力以及在核事故条件下的良好稳定性。UiO-66 在几项通过使用不同的接头和金属含量捕获 I 2的工作中得到了深刻的评估。将 I 2分子转化为 I 3 –这种多孔系统的内部结构在其他研究中得到了验证,但仍有待更好地阐明。理解这种转变可以改进用于捕获碘物质的材料,并保证长期更好地稳定这些污染物。出于这个原因,三个具有不同金属含量(Zr、Zr/Hf 和 Hf)的 UiO-67_NH 2 样品被用来捕获碘,并使用共振条件下和非共振条件下的拉曼光谱映射评估不同物种的特征( λ ex = 515、633 和 785 纳米)。UiO-67_NH 2 (Hf)化合物在室温下与气态I 2接触48 h后表现出最佳吸附能力,捕获3428 g·mol –1碘。另外两个样品UiO-67_NH 2 (Zr/Hf)和UiO-67_NH 2 (Zr)在相同条件下分别吸附了2835 g·mol –1和1658 g·mol –1 。I 2转变为 I 3 –由与“扰动”I 2和 I 3相关的条带的存在所证实–分别位于约 170 和 107 cm –1处。拉曼图谱表明,在与碘气流接触 48 小时后,单金属UiO-67_NH 2样品均显示出两种物质的均匀分布,而双金属样品则显示出具有不同浓度 I 的区域2和我3 –。这种效应与通过 UiO-67_NH 2微晶的 I 2扩散过程有关,单金属 UiO-67_NH 2样品的扩散速度更快,因为它们的晶体尺寸较小(对于 UiO-67_NH,φ ≈ 44 μm 和 φ ≈ 51 μm 2 (Hf) 和 UiO-67_NH 2 (Zr),分别与 UiO-67_NH 2 (Zr/Hf) 样品 (ϕ ≈ 140 μm) 相比。本文展示了 I 2和 I 3的空间分布——沿着 UiO-67_NH 2的晶体材料并将这些数据与两种物种的扩散过程相关联,提高了对 UiO 材料中碘转化和稳定机制的理解。
更新日期:2023-02-28
中文翻译:

拉曼映射作为评估 I2 和 I3 的工具——单晶 UiO-67_NH2(M) 上的扩散(M = Zr、Zr/Hf 或 Hf)
气态碘的捕获已被深入研究以试图减轻核能的危险。UiO 系列金属有机骨架 (MOF) 材料被认为是此类用途的最佳候选材料之一,因为它具有高比表面积、易于化学改性、强大的碘吸附能力以及在核事故条件下的良好稳定性。UiO-66 在几项通过使用不同的接头和金属含量捕获 I 2的工作中得到了深刻的评估。将 I 2分子转化为 I 3 –这种多孔系统的内部结构在其他研究中得到了验证,但仍有待更好地阐明。理解这种转变可以改进用于捕获碘物质的材料,并保证长期更好地稳定这些污染物。出于这个原因,三个具有不同金属含量(Zr、Zr/Hf 和 Hf)的 UiO-67_NH 2 样品被用来捕获碘,并使用共振条件下和非共振条件下的拉曼光谱映射评估不同物种的特征( λ ex = 515、633 和 785 纳米)。UiO-67_NH 2 (Hf)化合物在室温下与气态I 2接触48 h后表现出最佳吸附能力,捕获3428 g·mol –1碘。另外两个样品UiO-67_NH 2 (Zr/Hf)和UiO-67_NH 2 (Zr)在相同条件下分别吸附了2835 g·mol –1和1658 g·mol –1 。I 2转变为 I 3 –由与“扰动”I 2和 I 3相关的条带的存在所证实–分别位于约 170 和 107 cm –1处。拉曼图谱表明,在与碘气流接触 48 小时后,单金属UiO-67_NH 2样品均显示出两种物质的均匀分布,而双金属样品则显示出具有不同浓度 I 的区域2和我3 –。这种效应与通过 UiO-67_NH 2微晶的 I 2扩散过程有关,单金属 UiO-67_NH 2样品的扩散速度更快,因为它们的晶体尺寸较小(对于 UiO-67_NH,φ ≈ 44 μm 和 φ ≈ 51 μm 2 (Hf) 和 UiO-67_NH 2 (Zr),分别与 UiO-67_NH 2 (Zr/Hf) 样品 (ϕ ≈ 140 μm) 相比。本文展示了 I 2和 I 3的空间分布——沿着 UiO-67_NH 2的晶体材料并将这些数据与两种物种的扩散过程相关联,提高了对 UiO 材料中碘转化和稳定机制的理解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号