当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
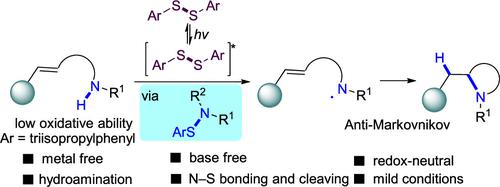
Photoinduced Disulfide-Catalyzed Intramolecular Anti-Markovnikov Hydroamination through in Situ N–S Species
Organic Letters ( IF 4.9 ) Pub Date : 2023-02-28 , DOI: 10.1021/acs.orglett.3c00508 Guoxiang Zhang 1 , Hui He 2 , Xiaoxiao Chen 1 , Shao-Fei Ni 2 , Rong Zeng 1
Organic Letters ( IF 4.9 ) Pub Date : 2023-02-28 , DOI: 10.1021/acs.orglett.3c00508 Guoxiang Zhang 1 , Hui He 2 , Xiaoxiao Chen 1 , Shao-Fei Ni 2 , Rong Zeng 1
Affiliation
|
The photoinduced anti-Markovnikov hydroamination of olefins typically required photocatalysts with a high oxidative ability to initiate the single-electron process. Herein, we alternatively utilize bis(2,4,6-triisopropylphenyl) disulfide, an inexpensive reagent with relatively low oxidative ability, as a photo and hydrogen atom transfer catalyst to achieve intramolecular hydroamination. The mechanistic studies as well as the DFT calculations are consistent with a novel process involving N-centered radical generation through the homolysis of the in situ formed N–S species and subsequent cyclization. An array of diverse nitrogen-containing cycles could be obtained.
中文翻译:

通过原位 N-S 物种光诱导二硫化物催化的分子内反马尔可夫尼科夫加氢胺化
烯烃的光诱导反马尔可夫尼科夫加氢胺化通常需要具有高氧化能力的光催化剂来引发单电子过程。在此,我们交替使用双(2,4,6-三异丙基苯基)二硫化物,一种氧化能力相对较低的廉价试剂,作为光和氢原子转移催化剂来实现分子内加氢胺化。机理研究以及 DFT 计算与涉及通过原位形成的 N-S 物种均裂和随后的环化产生 N 中心自由基的新过程一致。可以获得一系列不同的含氮循环。
更新日期:2023-02-28
中文翻译:

通过原位 N-S 物种光诱导二硫化物催化的分子内反马尔可夫尼科夫加氢胺化
烯烃的光诱导反马尔可夫尼科夫加氢胺化通常需要具有高氧化能力的光催化剂来引发单电子过程。在此,我们交替使用双(2,4,6-三异丙基苯基)二硫化物,一种氧化能力相对较低的廉价试剂,作为光和氢原子转移催化剂来实现分子内加氢胺化。机理研究以及 DFT 计算与涉及通过原位形成的 N-S 物种均裂和随后的环化产生 N 中心自由基的新过程一致。可以获得一系列不同的含氮循环。






































 京公网安备 11010802027423号
京公网安备 11010802027423号