Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2023-02-28 , DOI: 10.1016/j.bmcl.2023.129205 Byung-Nam Kang 1 , Hong-Jun Kang 2 , Sunjoo Kim 2 , Jungwoo Lee 2 , Jinwoo Lee 2 , Hee-Jin Jeong 2 , Seeun Jeon 2 , Youngdo Shin 2 , Cheolhwan Yoon 2 , Cheolkyu Han 3 , Jeongbeob Seo 2 , Jaesook Yun 4
|
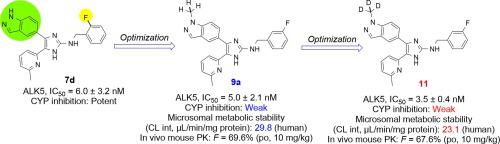
Specific inhibition of ALK5 provides a novel method for controlling the development of cancers and fibrotic diseases. In this work, a novel series of N-(3-fluorobenzyl)-4-(1-(methyl-d3)-1H-indazol-5-yl)-5-(6-methylpyridin-2-yl)-1H-imidazol-2-amine (11), a potential clinical candidate, was synthesized by strategic incorporation of deuterium at potential metabolic soft spots and identified as ALK5 inhibitors. This compound has a low potential for CYP-mediated drug-drug interactions as a CYP450 inhibitor (IC50 = >10 μM) and showed potent inhibitory effects in cellular assay (IC50 = 3.5 ± 0.4 nM). The pharmacokinetic evaluation of 11 in mice demonstrated moderate clearance (29.0 mL/min/kg) and also revealed high oral bioavailability in mice (F = 67.6%).
中文翻译:

N-(3-氟苄基)-4-(1-(methyl-d3)-1H-indazol-5-yl)-5-(6-methylpyridin-2-yl)-1H-imidazol-2的合成及生物学评价-胺作为一种新型、有效的 ALK5 受体抑制剂
特异性抑制 ALK5 为控制癌症和纤维化疾病的发展提供了一种新方法。在这项工作中,一系列新的N -(3-fluorobenzyl)-4-(1-(methyl-d 3 )-1 H -indazol-5-yl)-5-(6-methylpyridin-2-yl)- 1 H -imidazol-2-amine ( 11 ) 是一种潜在的临床候选药物,通过在潜在代谢软点处战略性掺入氘合成,并被鉴定为 ALK5 抑制剂。该化合物作为 CYP450 抑制剂 (IC 50 = >10 μM) 对 CYP 介导的药物-药物相互作用具有低潜力,并在细胞测定中显示出有效的抑制作用 (IC 50 = 3.5 ± 0.4 nM)。11的药代动力学评价在小鼠中表现出适度的清除率 (29.0 mL/min/kg),并且在小鼠中还显示出高口服生物利用度 ( F = 67.6%)。






























 京公网安备 11010802027423号
京公网安备 11010802027423号