Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2023-02-28 , DOI: 10.1016/j.jhazmat.2023.131103 Zhu Wang 1 , Kunjie Hou 2 , Fei Chen 3 , Shanshan Zhang 1 , Zhoujie Pi 1 , Li He 1 , Shengjie Chen 1 , Xiaoming Li 1 , Qi Yang 1
|
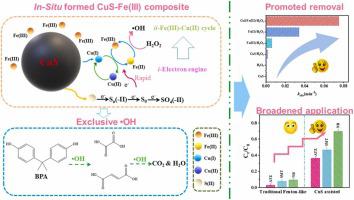
The conventional Fenton-like system (Fe(III)/H2O2) is severely limited by the inferior activity of Fe(III) on H2O2 activation to produce highly active species and the sluggish regeneration rate of Fe(II). This work significantly enhanced the oxidative breakdown of the target organic contaminant bisphenol A (BPA) by Fe(III)/H2O2 by introducing cheap CuS at a low dose of 50 mg/L. The BPA removal (20 mg/L) in CuS/Fe(III)/H2O2 system reached 89.5 % within 30 min under the optimal conditions: CuS dosage 50 mg/L, Fe(III) concentration 0.05 mM, H2O2 concentration 0.5 mM and pH 5.6. Compared to CuS/H2O2 and Fe(III)/H2O2 systems, the reaction constants had a 47- and 12.3-fold enhancement, respectively. Even compared with the conventional Fe(II)/H2O2 system, the kinetic constant also increased more than twice, further confirming the distinctive superiority of constructed system. Element species change analyses showed that Fe(III) in solution was adsorbed onto the CuS surface, and then Fe(III) was rapidly reduced by Cu(I) in the CuS lattice. Combining CuS and Fe(III) (in-situ formed CuS-Fe(III) composite) created a robust co-effect on the activation of H2O2. Also, S(-II) and its derivatives, e.g., Sn2- and S0 (as an electron donor), could quickly reduce Cu(II) to Cu(I) and ultimately oxidize to the harmless product SO42-. Notably, a mere 50 μM of Fe(III) was sufficient to maintain enough regenerated Fe(II) to effectively activate H2O2 in CuS/Fe(III)/H2O2 system. In addition, such a system achieved a broad range of pH applications and was more suitable for real wastewater containing anions and natural organic matter. Scavenging tests, electron paramagnetic resonance (EPR), and probes further verified the critical role of •OH. This work provides a new approach to solving the problems of Fenton systems through a solid-liquid-interfacial system design and exhibits considerable application potential in wastewater decontamination.
中文翻译:

在 CuS 介导的固-液-界面类芬顿系统中有效去除有机污染物:双金属循环和硫物种的作用
传统的类芬顿体系(Fe(III)/H 2 O 2 )严重受限于Fe(III)对H 2 O 2活化产生高活性物种的较差活性以及Fe(II)的缓慢再生速率. 这项工作通过以 50 mg/L 的低剂量引入廉价的 CuS,显着增强了 Fe(III)/H 2 O 2对目标有机污染物双酚 A (BPA) 的氧化分解。在CuS/Fe(III)/H 2 O 2体系中BPA去除率(20 mg/L)在30分钟内达到89.5%,最佳条件为:CuS用量50 mg/L,Fe(III)浓度0.05 mM,H 2氧2浓度 0.5 mM 和 pH 5.6。与CuS/H 2 O 2和Fe(III)/H 2 O 2系统相比,反应常数分别提高了47 倍和12.3 倍。即使与传统的Fe(II)/H 2 O 2体系相比,动力学常数也增加了两倍以上,进一步证实了所构建体系的独特优势。元素物种变化分析表明,溶液中的 Fe(III) 被吸附到 CuS 表面,然后 Fe(III) 被 CuS 晶格中的 Cu(I) 快速还原。结合 CuS 和 Fe(III)(原位形成的 CuS-Fe(III) 复合材料)对 H 2 O 2的活化产生强大的协同效应. 此外,S(-II) 及其衍生物,例如 S n 2-和 S 0(作为电子供体),可以快速将 Cu(II) 还原为 Cu(I),并最终氧化为无害产物 SO 4 2- . 值得注意的是,仅 50 μM 的 Fe(III) 就足以维持足够的再生 Fe(II) 以有效激活CuS/Fe(III)/H 2 O 2中的 H 2 O 2系统。此外,该系统实现了广泛的 pH 应用范围,更适用于含有阴离子和天然有机物的实际废水。清除试验、电子顺磁共振(EPR) 和探针进一步验证了•OH 的关键作用。这项工作通过固-液-界面系统设计为解决芬顿系统的问题提供了一种新方法,并在废水净化中展现出相当大的应用潜力。













































 京公网安备 11010802027423号
京公网安备 11010802027423号