Nano Research ( IF 9.5 ) Pub Date : 2023-02-27 , DOI: 10.1007/s12274-023-5375-2 Li Wang , Liang Xiao , Zhengyang Zhao , Kai Zhong , Weiliang Zhu , Hao Liu , Xiaoqiu Li
|
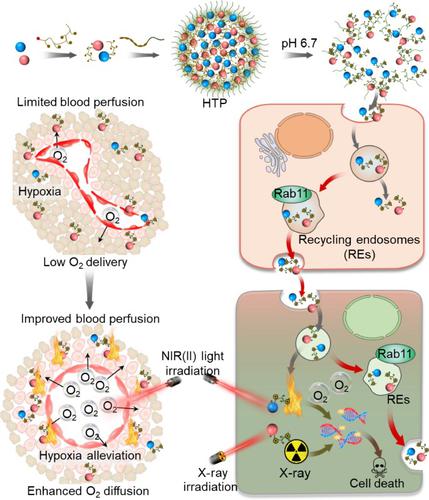
As a standard cancer treatment method, radiotherapy (RT) has cured or alleviated over half cancer bearing patients worldwide more than 100 years. However, the therapeutic outcome is seriously hindered by the resistant tumor microenvironment (TME). Hypoxia is a critical factor of vicious TME that causes radiation resistance owing to the insufficiency of oxygen for DNA damage maintenance. Moreover, severe vascular dysfunction and pyknomorphic extracellular matrix (ECM) in deep tumor tissues substantially limit radiosensitizer penetration and oxygen diffusion from vessels into tightly packed tumor core. In this study, we develop a hybrid transcytosis nanopomegranate (HTP) with high transcytosis potential in response to TME condition. HTP is architected by self-assembly of small CuS and Au nanoparticles (NPs) at normal physiological condition. HTP can rapidly collapse to transcytosis NPs (CuS and Au NPs) in TME with cationized surface, which enables excellent transcytosis potential and effectively elevates the penetration of CuS and Au into deep tumor tissues. Following the second near-infrared (NIR(II)) biowindow laser irradiation, CuS heats the tumor and enhances blood perfusion, eliciting tumor hypoxia alleviation and DNA damage aggravation. Moreover, Au NPs enriched in deep tumor tissues effectively sensitize radio-therapeutic response. Our study provides a new and potential nano-platform to ameliorate tumor hypoxia and sensitize deep tumor tissue radiotherapy.
中文翻译:

杂交转胞吞纳米石榴用于在深部肿瘤组织中敏化乳腺癌放疗
作为一种标准的癌症治疗方法,放疗(RT)在全球已有100多年的历史,治愈或减轻了一半以上的癌症患者。然而,耐药性肿瘤微环境 (TME) 严重阻碍了治疗结果。缺氧是恶性 TME 的关键因素,由于维持 DNA 损伤的氧气不足,会导致辐射抵抗。此外,深部肿瘤组织中严重的血管功能障碍和致密细胞外基质 (ECM) 显着限制了放射增敏剂的渗透和氧从血管扩散到紧密堆积的肿瘤核心。在这项研究中,我们开发了一种混合转胞吞纳米石榴 (HTP),它具有响应 TME 条件的高转胞吞作用潜力。HTP 是通过在正常生理条件下自组装小的 CuS 和 Au 纳米粒子 (NPs) 构建的。HTP 可以在表面阳离子化的 TME 中快速塌陷为转胞吞 NPs(CuS 和 Au NPs),这具有出色的转胞吞作用潜力,并有效提高 CuS 和 Au 对深部肿瘤组织的渗透。在第二次近红外 (NIR(II)) 生物窗口激光照射后,CuS 加热肿瘤并增强血液灌注,从而引起肿瘤缺氧缓解和 DNA 损伤加重。此外,富集在深部肿瘤组织中的 Au NPs 有效地提高了放射治疗反应的敏感性。我们的研究提供了一个新的和潜在的纳米平台来改善肿瘤缺氧和提高深部肿瘤组织放疗的敏感性。在第二次近红外 (NIR(II)) 生物窗口激光照射后,CuS 加热肿瘤并增强血液灌注,从而引起肿瘤缺氧缓解和 DNA 损伤加重。此外,富集在深部肿瘤组织中的 Au NPs 有效地提高了放射治疗反应的敏感性。我们的研究提供了一个新的和潜在的纳米平台来改善肿瘤缺氧和提高深部肿瘤组织放疗的敏感性。在第二次近红外 (NIR(II)) 生物窗口激光照射后,CuS 加热肿瘤并增强血液灌注,从而引起肿瘤缺氧缓解和 DNA 损伤加重。此外,富集在深部肿瘤组织中的 Au NPs 有效地提高了放射治疗反应的敏感性。我们的研究提供了一个新的和潜在的纳米平台来改善肿瘤缺氧和提高深部肿瘤组织放疗的敏感性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号