当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanical Interlocking Enhances the Electrocatalytic Oxygen Reduction Activity and Selectivity of Molecular Copper Complexes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-02-28 , DOI: 10.1021/jacs.2c10988 Xiaoyong Mo, Yulin Deng, Samuel Kin-Man Lai, Xutao Gao, Hung-Ling Yu, Kam-Hung Low, Zhengxiao Guo, Heng-Liang Wu, Ho Yu Au-Yeung, Edmund C. M. Tse
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-02-28 , DOI: 10.1021/jacs.2c10988 Xiaoyong Mo, Yulin Deng, Samuel Kin-Man Lai, Xutao Gao, Hung-Ling Yu, Kam-Hung Low, Zhengxiao Guo, Heng-Liang Wu, Ho Yu Au-Yeung, Edmund C. M. Tse
|
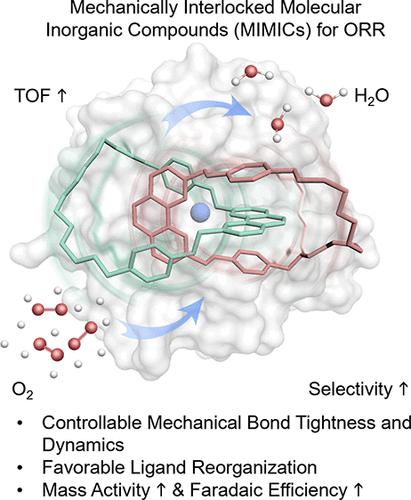
Efficient O2 reduction reaction (ORR) for selective H2O generation enables advanced fuel cell technology. Nonprecious metal catalysts are viable and attractive alternatives to state-of-the-art Pt-based materials that are expensive. Cu complexes inspired by Cu-containing O2 reduction enzymes in nature are yet to reach their desired ORR catalytic performance. Here, the concept of mechanical interlocking is introduced to the ligand architecture to enforce dynamic spatial restriction on the Cu coordination site. Interlocked catenane ligands could govern O2 binding mode, promote electron transfer, and facilitate product elimination. Our results show that ligand interlocking as a catenane steers the ORR selectivity to H2O as the major product via the 4e– pathway, rivaling the selectivity of Pt, and boosts the onset potential by 130 mV, the mass activity by 1.8 times, and the turnover frequency by 1.5 fold as compared to the noninterlocked counterpart. Our Cu catenane complex represents one of the first examples to take advantage of mechanical interlocking to afford electrocatalysts with enhanced activity and selectivity. The mechanistic insights gained through this integrated experimental and theoretical study are envisioned to be valuable not just to the area of ORR energy catalysis but also with broad implications on interlocked metal complexes that are of critical importance to the general fields in redox reactions involving proton-coupled electron transfer steps.
中文翻译:

机械联锁增强分子铜配合物的电催化氧还原活性和选择性
用于选择性生成 H 2 O 的高效 O 2还原反应 (ORR)使先进的燃料电池技术成为可能。非贵金属催化剂是最先进的昂贵 Pt 基材料的可行且有吸引力的替代品。受自然界含 Cu O 2还原酶启发的 Cu 络合物尚未达到其所需的 ORR 催化性能。在这里,机械联锁的概念被引入到配体结构中,以对 Cu 配位点实施动态空间限制。互锁的链烷配体可以控制 O 2结合模式,促进电子转移,并促进产物消除。我们的结果表明,作为链烷的配体互锁可控制 ORR 对 H 2的选择性O 作为 4e-通路的主要产物,与 Pt 的选择性相媲美,与非互锁对应物相比,起始电位提高 130 mV,质量活性提高 1.8 倍,转换频率提高 1.5 倍。我们的铜链烷络合物代表了第一个利用机械联锁来提供具有增强活性和选择性的电催化剂的例子。通过这项综合实验和理论研究获得的机理见解被认为不仅对 ORR 能量催化领域有价值,而且对联锁金属配合物具有广泛的意义,这些金属配合物对于涉及质子耦合的氧化还原反应的一般领域至关重要电子转移步骤。
更新日期:2023-02-28
中文翻译:

机械联锁增强分子铜配合物的电催化氧还原活性和选择性
用于选择性生成 H 2 O 的高效 O 2还原反应 (ORR)使先进的燃料电池技术成为可能。非贵金属催化剂是最先进的昂贵 Pt 基材料的可行且有吸引力的替代品。受自然界含 Cu O 2还原酶启发的 Cu 络合物尚未达到其所需的 ORR 催化性能。在这里,机械联锁的概念被引入到配体结构中,以对 Cu 配位点实施动态空间限制。互锁的链烷配体可以控制 O 2结合模式,促进电子转移,并促进产物消除。我们的结果表明,作为链烷的配体互锁可控制 ORR 对 H 2的选择性O 作为 4e-通路的主要产物,与 Pt 的选择性相媲美,与非互锁对应物相比,起始电位提高 130 mV,质量活性提高 1.8 倍,转换频率提高 1.5 倍。我们的铜链烷络合物代表了第一个利用机械联锁来提供具有增强活性和选择性的电催化剂的例子。通过这项综合实验和理论研究获得的机理见解被认为不仅对 ORR 能量催化领域有价值,而且对联锁金属配合物具有广泛的意义,这些金属配合物对于涉及质子耦合的氧化还原反应的一般领域至关重要电子转移步骤。































 京公网安备 11010802027423号
京公网安备 11010802027423号