当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamics, Kinetics, and Photochemistry of β-Strand Association and Dissociation in a Split-GFP System
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-11-16 , DOI: 10.1021/ja207985w
Keunbong Do 1 , Steven G Boxer
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-11-16 , DOI: 10.1021/ja207985w
Keunbong Do 1 , Steven G Boxer
Affiliation
|
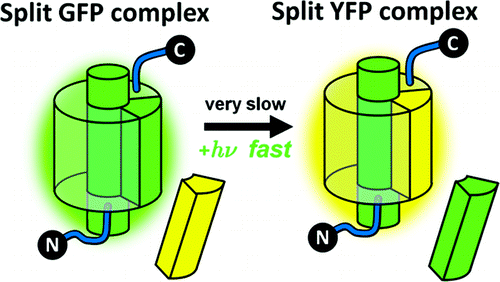
Truncated green fluorescent protein (GFP) that is refolded after removing the 10th β-strand can readily bind to a synthetic strand to recover the absorbance and fluorescence of the whole protein. This allows rigorous experimental determination of thermodynamic and kinetic parameters of the split system including the equilibrium constant and the association/dissociation rates, which enables residue-specific analysis of peptide-protein interactions. The dissociation rate of the noncovalently bound strand is observed by strand exchange that is accompanied by a color change, and surprisingly, the rate is greatly enhanced by light irradiation. This peptide-protein photodissociation is a very unusual phenomenon and can potentially be useful for introducing spatially and temporally well-defined perturbations to biological systems as a genetically encoded caged protein.
中文翻译:

Split-GFP 系统中 β 链结合和解离的热力学、动力学和光化学
去除第 10 条 β 链后重新折叠的截短绿色荧光蛋白 (GFP) 可以很容易地与合成链结合,以恢复整个蛋白质的吸光度和荧光。这允许严格的实验确定分裂系统的热力学和动力学参数,包括平衡常数和缔合/解离速率,从而能够对肽-蛋白质相互作用进行残基特异性分析。通过伴随颜色变化的链交换观察非共价结合链的解离速率,令人惊讶的是,光照射大大提高了该速率。这种肽-蛋白质光解离是一种非常不寻常的现象,作为基因编码的笼蛋白,可能有助于向生物系统引入空间和时间上明确的扰动。
更新日期:2011-11-16
中文翻译:

Split-GFP 系统中 β 链结合和解离的热力学、动力学和光化学
去除第 10 条 β 链后重新折叠的截短绿色荧光蛋白 (GFP) 可以很容易地与合成链结合,以恢复整个蛋白质的吸光度和荧光。这允许严格的实验确定分裂系统的热力学和动力学参数,包括平衡常数和缔合/解离速率,从而能够对肽-蛋白质相互作用进行残基特异性分析。通过伴随颜色变化的链交换观察非共价结合链的解离速率,令人惊讶的是,光照射大大提高了该速率。这种肽-蛋白质光解离是一种非常不寻常的现象,作为基因编码的笼蛋白,可能有助于向生物系统引入空间和时间上明确的扰动。

































 京公网安备 11010802027423号
京公网安备 11010802027423号