当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
MnOOH-Catalyzed Autoxidation of Glutathione for Reactive Oxygen Species Production and Nanocatalytic Tumor Innate Immunotherapy
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-02-27 , DOI: 10.1021/jacs.2c12942
Piao Zhu 1 , Yinying Pu 2 , Min Wang 3 , Wencheng Wu 3 , Huanlong Qin 4 , Jianlin Shi 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-02-27 , DOI: 10.1021/jacs.2c12942
Piao Zhu 1 , Yinying Pu 2 , Min Wang 3 , Wencheng Wu 3 , Huanlong Qin 4 , Jianlin Shi 1, 3
Affiliation
|
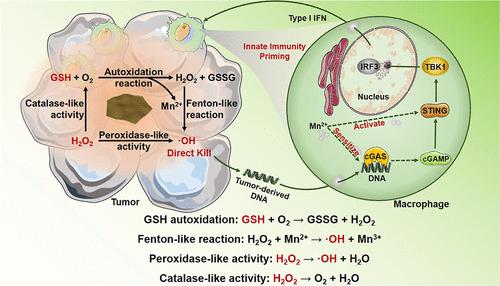
The antioxidant system, signed with reduced glutathione (GSH) overexpression, is the key weapon for tumor to resist the attack by reactive oxygen species (ROS). Counteracting the ROS depletion by GSH is an effective strategy to guarantee the antitumor efficacy of nanocatalytic therapy. However, simply reducing the concentration of GSH does not sufficiently improve tumor response to nanocatalytic therapy intervention. Herein, a well-dispersed MnOOH nanocatalyst is developed to catalyze GSH autoxidation and peroxidase-like reaction concurrently and respectively to promote GSH depletion and H2O2 decomposition to produce abundant ROS such as hydroxyl radical (·OH), thereby generating a highly effective superadditive catalytic therapeutic efficacy. Such a therapeutic strategy that transforms endogenous “antioxidant” into “oxidant” may open a new avenue for the development of antitumor nanocatalytic medicine. Moreover, the released Mn2+ can activate and sensitize the cGAS-STING pathway to the damaged intratumoral DNA double-strands induced by the produced ROS to further promote macrophage maturation and M1-polarization, which will boost the innate immunotherapeutic efficacy. Resultantly, the developed simple MnOOH nanocatalytic medicine capable of simultaneously catalyzing GSH depletion and ROS generation, and mediating innate immune activation, holds great potential in the treatment of malignant tumors.
中文翻译:

MnOOH 催化谷胱甘肽自氧化产生活性氧和纳米催化肿瘤先天免疫治疗
以还原型谷胱甘肽(GSH)过表达为标志的抗氧化系统是肿瘤抵抗活性氧(ROS)攻击的关键武器。通过GSH抵消ROS消耗是保证纳米催化疗法抗肿瘤功效的有效策略。然而,简单地降低 GSH 的浓度并不能充分改善肿瘤对纳米催化治疗干预的反应。在此,开发了一种分散良好的 MnOOH 纳米催化剂,同时分别催化 GSH 自氧化和过氧化物酶样反应,以促进 GSH 消耗和 H 2 O 2分解产生丰富的ROS,如羟基自由基(·OH),从而产生高效的超加催化治疗效果。这种将内源性“抗氧化剂”转化为“氧化剂”的治疗策略可能为抗肿瘤纳米催化药物的发展开辟一条新途径。此外,释放的 Mn 2+可以激活cGAS-STING通路并使其对产生的ROS诱导的受损瘤内DNA双链敏感,进一步促进巨噬细胞成熟和M1极化,从而提高先天免疫治疗效果。因此,开发的简单的 MnOOH 纳米催化药物能够同时催化 GSH 消耗和 ROS 生成,并介导先天免疫激活,在治疗恶性肿瘤方面具有巨大潜力。
更新日期:2023-02-27
中文翻译:

MnOOH 催化谷胱甘肽自氧化产生活性氧和纳米催化肿瘤先天免疫治疗
以还原型谷胱甘肽(GSH)过表达为标志的抗氧化系统是肿瘤抵抗活性氧(ROS)攻击的关键武器。通过GSH抵消ROS消耗是保证纳米催化疗法抗肿瘤功效的有效策略。然而,简单地降低 GSH 的浓度并不能充分改善肿瘤对纳米催化治疗干预的反应。在此,开发了一种分散良好的 MnOOH 纳米催化剂,同时分别催化 GSH 自氧化和过氧化物酶样反应,以促进 GSH 消耗和 H 2 O 2分解产生丰富的ROS,如羟基自由基(·OH),从而产生高效的超加催化治疗效果。这种将内源性“抗氧化剂”转化为“氧化剂”的治疗策略可能为抗肿瘤纳米催化药物的发展开辟一条新途径。此外,释放的 Mn 2+可以激活cGAS-STING通路并使其对产生的ROS诱导的受损瘤内DNA双链敏感,进一步促进巨噬细胞成熟和M1极化,从而提高先天免疫治疗效果。因此,开发的简单的 MnOOH 纳米催化药物能够同时催化 GSH 消耗和 ROS 生成,并介导先天免疫激活,在治疗恶性肿瘤方面具有巨大潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号