当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electric Potential Distribution Inside the Electrolyte during High Voltage Electrolysis
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2023-02-23 , DOI: 10.1021/acs.jpcc.2c07873 Lukas Forschner 1 , Evelyn Artmann 1 , Timo Jacob 1 , Albert K. Engstfeld 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2023-02-23 , DOI: 10.1021/acs.jpcc.2c07873 Lukas Forschner 1 , Evelyn Artmann 1 , Timo Jacob 1 , Albert K. Engstfeld 1
Affiliation
|
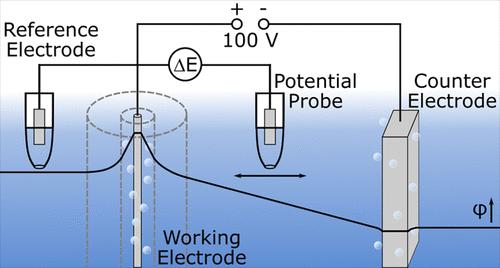
Applying an external potential difference between two electrodes leads to a voltage drop in an ion conducting electrolyte. This drop is particularly large in poorly conducting electrolytes and for high currents. Measuring the electrolyte potential is relevant in electrochemistry, e.g., bipolar electrochemistry, ohmic microscopy, or contact glow discharge electrolysis. Here, we study the course of the electrolyte potential during high voltage electrolysis in an electrolysis cell using two reversible hydrogen electrodes as reference electrodes, placed at different positions in the electrolyte. The electrolysis is performed with a Pt working and stainless steel counter electrode in a KOH solution. A computational COMSOL model is devised which supports the experimentally obtained potential distribution. The influence of the cell geometry on the electrolyte potentials is evaluated. Applying the knowledge of the potential distribution to the formation of a Au oxide surface structure produced during high voltage electrolysis, we find that the amount of oxide formed is related to the current rather than the applied voltage.
中文翻译:

高压电解过程中电解液内部的电势分布
在两个电极之间施加外部电势差会导致离子导电电解质中出现电压降。这种下降在导电性差的电解质和高电流中特别大。测量电解质电位与电化学相关,例如双极电化学、欧姆显微镜或接触辉光放电电解。在这里,我们使用两个可逆氢电极作为参考电极,放置在电解质中的不同位置,研究了电解槽中高压电解过程中电解质电位的过程。电解是在 KOH 溶液中使用 Pt 工作电极和不锈钢反电极进行的。设计了一个计算 COMSOL 模型,它支持通过实验获得的电位分布。评估了电池几何形状对电解质电位的影响。将电位分布的知识应用于高压电解过程中产生的 Au 氧化物表面结构的形成,我们发现形成的氧化物量与电流有关,而不是与施加的电压有关。
更新日期:2023-02-23
中文翻译:

高压电解过程中电解液内部的电势分布
在两个电极之间施加外部电势差会导致离子导电电解质中出现电压降。这种下降在导电性差的电解质和高电流中特别大。测量电解质电位与电化学相关,例如双极电化学、欧姆显微镜或接触辉光放电电解。在这里,我们使用两个可逆氢电极作为参考电极,放置在电解质中的不同位置,研究了电解槽中高压电解过程中电解质电位的过程。电解是在 KOH 溶液中使用 Pt 工作电极和不锈钢反电极进行的。设计了一个计算 COMSOL 模型,它支持通过实验获得的电位分布。评估了电池几何形状对电解质电位的影响。将电位分布的知识应用于高压电解过程中产生的 Au 氧化物表面结构的形成,我们发现形成的氧化物量与电流有关,而不是与施加的电压有关。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号