Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2023-02-25 , DOI: 10.1016/j.bioorg.2023.106446 Yongxi Dong 1 , Jun Lu 1 , Shanhui Zhang 1 , Lina Chen 1 , Jinlan Wen 1 , Fang Wang 1 , Yongqing Mao 1 , Lei Li 2 , Jiquan Zhang 1 , Shanggao Liao 1 , Li Dong 1
|
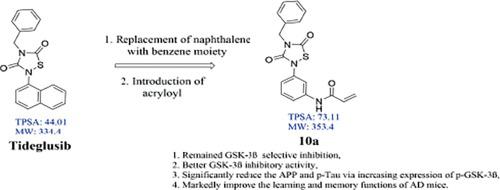
Tideglusib is a non-competitive GSK-3β inhibitor which contain 1,2,4-thiadiazolidine-3,5-dione moiety, and now mainly used for progressive supranuclear palsy due to the lack of some primary cognitive endpoints and secondary endpoints in a phase IIb trail for Alzheimer's disease. Additionally, insufficient evidence exists to support that there are obvious covalent bonds between Tideglusib and GSK-3β. Targeted covalent inhibition strategy could improve the binding efficiency, selectivity and duration of kinase inhibitors. Based on the above premise, two series of targeted compounds with acryloyl warheads were designed and synthesized. The kinase inhibitory activity of the selected compound 10a with better neuroprotective effect improved 2.7 fold than that of Tideglusib. After the preliminary screening of GSK-3β inhibition and neuroprotective activity, the mechanism action of the selected compound 10a was investigated in vitro and in vivo. The results confirmed that 10a with excellent selectivity among the whole tested kinases could significantly reduce the expressions of APP and p-Tau via increasing the level of p-GSK-3β. The pharmacodynamic assay in vivo showed that 10a could markedly improve the learning and memory functions in AD mice induced by AlCl3 combined with d-galactose. At the same time, the damage of hippocampal neurons in AD mice was obviously reduced. Accordingly, the introduction of acryloyl warheads could increase the GSK-3β inhibitory activity of 1,2,4-thiadiazolidine-3,5-dione derivatives, and the selected compound 10a deserves further research as an effective GSK-3β inhibitor for the potential treatment of AD.
中文翻译:

1,2,4-thiadiazolidine-3,5-dione 衍生物作为治疗阿尔茨海默病的潜在 GSK-3β 抑制剂的设计、合成和生物评价
Tideglusib 是一种非竞争性 GSK-3β 抑制剂,含有 1,2,4-thiadiazolidine-3,5-dione 部分,由于缺乏一些主要认知终点和次要终点,目前主要用于进行性核上性麻痹阿尔茨海默病的 IIb 试验。此外,没有足够的证据支持 Tideglusib 和 GSK-3β 之间存在明显的共价键。靶向共价抑制策略可以提高激酶抑制剂的结合效率、选择性和持续时间。基于以上前提,设计合成了两个系列的丙烯酰弹头靶向化合物。所选化合物10a的激酶抑制活性具有更好的神经保护作用,比Tideglusib提高2.7倍。在初步筛选出 GSK-3β 抑制和神经保护活性后,对所选化合物10a的体外和体内作用机制进行了研究。结果证实,在所有测试的激酶中具有优异选择性的10a可以通过增加 p-GSK-3β 的水平显着降低 APP 和 p-Tau 的表达。体内药效学实验表明,10a可显着改善AlCl 3联合d诱导的AD小鼠学习记忆功能。-半乳糖。同时,AD小鼠海马神经元的损伤明显减轻。因此,丙烯酰弹头的引入可以增加 1,2,4-噻二唑烷-3,5-二酮衍生物的 GSK-3β 抑制活性,所选化合物 10a 作为有效的 GSK-3β 抑制剂值得进一步研究,用于潜在治疗公元。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号