Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2023-02-25 , DOI: 10.1016/j.apcatb.2023.122529 Rongqiang Yin , Jianjun Chen , Liang Shan , Jianqiang Shi , Kun Yang , Hao Liu , Junhua Li
|
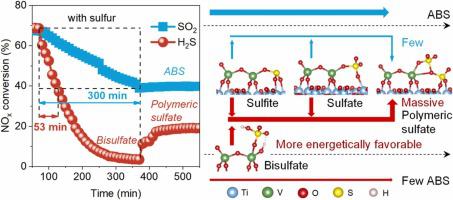
Selective catalytic reduction (SCR) over V2O5/TiO2-based catalysts is the most efficient technology to remove nitrogen oxides from stationary sources, where H2S coexists with NOx under variable combustion conditions. For the first time, the poisoning effect of H2S was observed to be almost six-fold that of SO2. Catalyst deactivation under SO2 was mainly caused by ammonium bisulfate while sulfates, especially polymeric sulfate, were the main contributors to H2S poisoning. Theoretical calculations showed that the generation of sulfates was much more energetically favorable under H2S than under SO2, resulting in massive deposition of sulfates and serious damage to vanadyl (V O). Polymeric sulfate could inhibit the charge transfer between the occupied and unoccupied orbitals of V
O). Polymeric sulfate could inhibit the charge transfer between the occupied and unoccupied orbitals of V O and suppress NH3 activation, thereby significantly restraining SCR activity. Furthermore, unstable sulfur species were shown to be responsible for the temporary deactivation during H2S poisoning. This work provides crucial information for solving catalyst deactivation.
O and suppress NH3 activation, thereby significantly restraining SCR activity. Furthermore, unstable sulfur species were shown to be responsible for the temporary deactivation during H2S poisoning. This work provides crucial information for solving catalyst deactivation.
中文翻译:

NH3 选择性催化还原 NOx 过程中 V2O5/TiO2 在 H2S 或 SO2 下的失活率和机理的显着差异
基于 V 2 O 5 /TiO 2的催化剂的选择性催化还原 (SCR)是从固定来源去除氮氧化物的最有效技术,其中 H 2 S在可变燃烧条件下与 NO x共存。首次观察到 H 2 S 的中毒作用几乎是 SO 2的六倍。催化剂在SO 2下的失活主要由硫酸氢铵引起,而硫酸盐,尤其是聚合硫酸盐,是H 2 S中毒的主要原因。理论计算表明,在 H 2下,硫酸盐的生成在能量上更为有利S 低于 SO 2,导致硫酸盐大量沉积,严重破坏氧钒(VO  )。
)。 聚合硫酸盐可以抑制V O占据和未占据轨道之间的电荷转移并抑制NH 3活化,从而显着抑制SCR活性。此外,不稳定的硫物质被证明是造成 H 2 S 中毒期间暂时失活的原因。这项工作为解决催化剂失活问题提供了重要信息。
聚合硫酸盐可以抑制V O占据和未占据轨道之间的电荷转移并抑制NH 3活化,从而显着抑制SCR活性。此外,不稳定的硫物质被证明是造成 H 2 S 中毒期间暂时失活的原因。这项工作为解决催化剂失活问题提供了重要信息。






























 京公网安备 11010802027423号
京公网安备 11010802027423号