当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic study of homoleptic trisamidolanthanide-catalyzed aldehyde and ketone hydroboration. Chemically non-innocent ligand participation
Chemical Science ( IF 7.6 ) Pub Date : 2023-02-24 , DOI: 10.1039/d2sc06442a Jacob O Rothbaum 1 , Alessandro Motta 2 , Yosi Kratish 1 , Tobin J Marks 1
Chemical Science ( IF 7.6 ) Pub Date : 2023-02-24 , DOI: 10.1039/d2sc06442a Jacob O Rothbaum 1 , Alessandro Motta 2 , Yosi Kratish 1 , Tobin J Marks 1
Affiliation
|
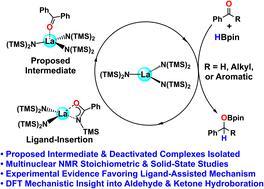
Carbonyl bond hydroboration is a valuable synthetic route to functionalized alcohols but relies on sometimes unselective and sluggish reagents. While rapid and selective aldehyde and ketone hydroboration mediated by trisamidolanthanide catalysts is known, the origin of the selectivity is not well-understood and is the subject of this contribution. Here the aldehyde and ketone HBpin hydroboration reaction mechanisms catalyzed by La[N(SiMe3)2]3 are investigated both experimentally and theoretically. The results support initial carbonyl oxygen coordination to the acidic La center, followed by intramolecular ligand-assisted hydroboration of the carbonyl moiety by bound HBpin. Interestingly, ketone hydroboration has a higher energetic barrier than that of aldehydes due to the increased steric encumbrance and decreased electrophilicity. Utilizing NMR spectroscopy and X-ray diffraction, a bidentate acylamino lanthanide complex associated with the aldehyde hydroboration is isolated and characterized, consistent with the relative reaction rates. Furthermore, an aminomonoboronate–lanthanide complex produced when the La catalyst is exposed to excess HBpin is isolated and characterized by X-ray diffraction, illuminating unusual aminomonoboronate coordination. These results shed new light on the origin of the catalytic activity patterns, reveal a unique ligand-assisted hydroboration pathway, and uncover previously unknown catalyst deactivation pathways.
中文翻译:

均配三氨基镧系元素催化醛酮硼氢化反应的机理研究。化学上非无辜的配体参与
羰基键硼氢化是一种有价值的功能化醇合成途径,但有时依赖于非选择性和缓慢的试剂。虽然由三氨基镧系元素催化剂介导的快速和选择性醛和酮硼氢化反应是已知的,但选择性的起源尚不清楚,并且是该贡献的主题。La[N(SiMe 3 ) 2 ] 3催化的醛和酮 HBpin 硼氢化反应机理在实验和理论上都进行了研究。结果支持初始羰基氧与酸性 La 中心的配位,然后通过结合的 HBpin 进行羰基部分的分子内配体辅助硼氢化。有趣的是,由于空间阻碍增加和亲电性降低,酮硼氢化反应比醛类具有更高的能量势垒。利用 NMR 光谱和 X 射线衍射,分离并表征了与醛氢硼化反应相关的双齿酰基氨基镧系元素配合物,与相对反应速率一致。此外,当 La 催化剂暴露于过量 HBpin 时产生的氨基单硼酸盐 - 镧系元素络合物被分离并通过 X 射线衍射表征,阐明了不寻常的氨基单硼酸盐配位。
更新日期:2023-03-01
中文翻译:

均配三氨基镧系元素催化醛酮硼氢化反应的机理研究。化学上非无辜的配体参与
羰基键硼氢化是一种有价值的功能化醇合成途径,但有时依赖于非选择性和缓慢的试剂。虽然由三氨基镧系元素催化剂介导的快速和选择性醛和酮硼氢化反应是已知的,但选择性的起源尚不清楚,并且是该贡献的主题。La[N(SiMe 3 ) 2 ] 3催化的醛和酮 HBpin 硼氢化反应机理在实验和理论上都进行了研究。结果支持初始羰基氧与酸性 La 中心的配位,然后通过结合的 HBpin 进行羰基部分的分子内配体辅助硼氢化。有趣的是,由于空间阻碍增加和亲电性降低,酮硼氢化反应比醛类具有更高的能量势垒。利用 NMR 光谱和 X 射线衍射,分离并表征了与醛氢硼化反应相关的双齿酰基氨基镧系元素配合物,与相对反应速率一致。此外,当 La 催化剂暴露于过量 HBpin 时产生的氨基单硼酸盐 - 镧系元素络合物被分离并通过 X 射线衍射表征,阐明了不寻常的氨基单硼酸盐配位。































 京公网安备 11010802027423号
京公网安备 11010802027423号