European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-02-23 , DOI: 10.1016/j.ejmech.2023.115222 Alexis Paquin 1 , Laurie Fortin 2 , Julie Girouard 2 , Carlos Reyes-Moreno 2 , Irina F Sevrioukova 3 , Gervais Bérubé 1
|
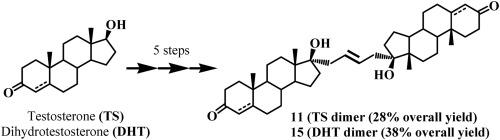
The synthesis of a 17α-linked C2-symmetric testosterone dimer and its dihydrotestosterone analog is reported. The dimers were synthesized using a short five-step reaction sequence with 28% and 38% overall yield for the testosterone and dihydrotestosterone dimer, respectively. The dimerization reaction was achieved by an olefin metathesis reaction with 2nd generation Hoveyda-Grubbs catalyst. The dimers and their corresponding 17α-allyl precursors were tested for the antiproliferative activity on androgen–dependent (LNCaP) and androgen–independent (PC3) prostate cancer cell lines. The effects on cells were compared with that of the antiandrogen cyproterone acetate (CPA). The results showed that the dimers were active on both cell lines, with an increased activity towards androgen–dependent LNCaP cells. However, the testosterone dimer (11) was fivefold more active than the dihydrotestosterone dimer (15), with an IC50 of 11.7 μM vs. 60.9 μM against LNCaP cells, respectively, and more than threefold more active than the reference drug CPA (IC50 of 40.7 μM). Likewise, studies on the interaction of new compounds with drug-metabolizing cytochrome P450 3A4 (CYP3A4) showed that 11 was a fourfold stronger inhibitor than 15 (IC50 of 3 μM and 12 μM, respectively). This suggests that changes in the chemical structure of sterol moieties and the manner of their linkage could largely affect both the antiproliferative activity of androgen dimers and their crossreactivity with CYP3A4.
中文翻译:

研究新的 C2 对称睾酮二聚体及其二氢睾酮类似物:合成、对前列腺癌细胞系的抗增殖活性以及与 CYP3A4 的相互作用
报道了 17α-连接的C2对称睾酮二聚体及其二氢睾酮类似物的合成。使用短的五步反应序列合成二聚体,睾酮和二氢睾酮二聚体的总产率分别为 28% 和 38%。二聚反应是通过第二代Hoveyda-Grubbs催化剂的烯烃复分解反应实现的。测试了二聚体及其相应的 17α-烯丙基前体对雄激素依赖性 (LNCaP) 和雄激素非依赖性 (PC3) 前列腺癌细胞系的抗增殖活性。将对细胞的影响与抗雄激素醋酸环丙孕酮(CPA)进行比较。结果表明,二聚体在两种细胞系上均具有活性,并且对雄激素依赖性 LNCaP 细胞的活性增强。然而,睾酮二聚体 ( 11 ) 的活性是二氢睾酮二聚体 ( 15 ) 的五倍,针对 LNCaP 细胞的 IC 50分别为 11.7 μM 和 60.9 μM,比参考药物 CPA 的活性高出三倍多 (IC 50 个40.7 μM)。同样,对新化合物与药物代谢细胞色素 P450 3A4 (CYP3A4) 相互作用的研究表明, 11是比15 强四倍的抑制剂( IC 50分别为 3 μM 和 12 μM)。这表明甾醇部分的化学结构及其连接方式的变化可能在很大程度上影响雄激素二聚体的抗增殖活性及其与 CYP3A4 的交叉反应性。































 京公网安备 11010802027423号
京公网安备 11010802027423号